Page 70 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 70
46 3 Monooxygenase-Catalyzed Redox Cascade Biotransformations
H O
2
O 2
O O
R 2
R 1 R 2 R 1 O
+
NADPH NADP
Phosphite
Phosphate
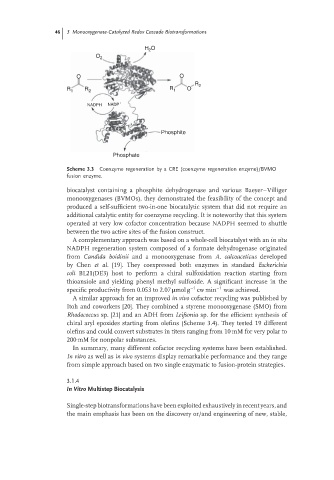
Scheme 3.3 Coenzyme regeneration by a CRE (coenzyme regeneration enzyme)/BVMO
fusion enzyme.
biocatalyst containing a phosphite dehydrogenase and various Baeyer–Villiger
monooxygenases (BVMOs), they demonstrated the feasibility of the concept and
produced a self-sufficient two-in-one biocatalytic system that did not require an
additional catalytic entity for coenzyme recycling. It is noteworthy that this system
operated at very low cofactor concentration because NADPH seemed to shuttle
between the two active sites of the fusion construct.
A complementary approach was based on a whole-cell biocatalyst with an in situ
NADPH regeneration system composed of a formate dehydrogenase originated
from Candida boidinii and a monooxygenase from A. calcoaceticus developed
by Chen et al. [19]. They coexpressed both enzymes in standard Escherichia
coli BL21(DE3) host to perform a chiral sulfoxidation reaction starting from
thioansiole and yielding phenyl methyl sulfoxide. A significant increase in the
specific productivity from 0.053 to 2.07 μmol g −1 cw min −1 was achieved.
A similar approach for an improved in vivo cofactor recycling was published by
Itoh and coworkers [20]. They combined a styrene monooxygenase (SMO) from
Rhodococcus sp. [21] and an ADH from Leifsonia sp. for the efficient synthesis of
chiral aryl epoxides starting from olefins (Scheme 3.4). They tested 19 different
olefins and could convert substrates in titers ranging from 10 mM for very polar to
200 mM for nonpolar substances.
In summary, many different cofactor recycling systems have been established.
In vitro as well as in vivo systems display remarkable performance and they range
from simple approach based on two single enzymatic to fusion-protein strategies.
3.1.4
In Vitro Multistep Biocatalysis
Single-step biotransformations have been exploited exhaustively in recent years, and
the main emphasis has been on the discovery or/and engineering of new, stable,