Page 252 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 252
BONE MECHANICS 229
Multiaxial failure properties of cortical bone
are not well understood, although it is clear that
simple isotropic and symmetrical criteria such
as the von Mises are not capable of describing
the multiaxial strength properties of this tissue.
The Tsai-Wu criterion, commonly used for
fiber-reinforced composite materials, has been
applied to cortical bone using both transversely
isotropic 31 and orthotropic 32 treatments. The
transversely isotropic case works quite well for
31
axial-shear-loading configurations, but neither
this case nor the orthotropic one has been vali-
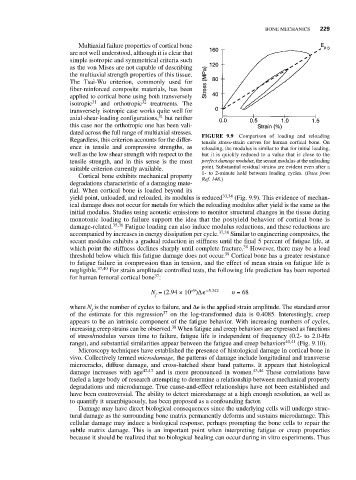
dated across the full range of multiaxial stresses. FIGURE 9.9 Comparison of loading and reloading
Regardless, this criterion accounts for the differ- tensile stress-strain curves for human cortical bone. On
ence in tensile and compressive strengths, as reloading, the modulus is similar to that for initial loading,
well as the low shear strength with respect to the but it is quickly reduced to a value that is close to the
tensile strength, and in this sense is the most perfect damage modulus, the secant modulus at the unloading
suitable criterion currently available. point. Substantial residual strains are evident even after a
1- to 2-minute hold between loading cycles. (Data from
Cortical bone exhibits mechanical property Ref. 148.)
degradations characteristic of a damaging mate-
rial. When cortical bone is loaded beyond its
yield point, unloaded, and reloaded, its modulus is reduced 33,34 (Fig. 9.9). This evidence of mechan-
ical damage does not occur for metals for which the reloading modulus after yield is the same as the
initial modulus. Studies using acoustic emissions to monitor structural changes in the tissue during
monotonic loading to failure support the idea that the postyield behavior of cortical bone is
damage-related. 35,36 Fatigue loading can also induce modulus reductions, and these reductions are
accompanied by increases in energy dissipation per cycle. 37,38 Similar to engineering composites, the
secant modulus exhibits a gradual reduction in stiffness until the final 5 percent of fatigue life, at
which point the stiffness declines sharply until complete fracture. 38 However, there may be a load
threshold below which this fatigue damage does not occur. 39 Cortical bone has a greater resistance
to fatigue failure in compression than in tension, and the effect of mean strain on fatigue life is
negligible. 37,40 For strain amplitude controlled tests, the following life prediction has been reported
37
for human femoral cortical bone :
−9
N = (2.94 × 10 )Δ −5.342 n = 68
f
where N is the number of cycles to failure, and Δ is the applied strain amplitude. The standard error
f
of the estimate for this regression 37 on the log-transformed data is 0.4085. Interestingly, creep
appears to be an intrinsic component of the fatigue behavior. With increasing numbers of cycles,
38
increasing creep strains can be observed. When fatigue and creep behaviors are expressed as functions
of stress/modulus versus time to failure, fatigue life is independent of frequency (0.2- to 2.0-Hz
range), and substantial similarities appear between the fatigue and creep behaviors 40,41 (Fig. 9.10).
Microscopy techniques have established the presence of histological damage in cortical bone in
vivo. Collectively termed microdamage, the patterns of damage include longitudinal and transverse
microcracks, diffuse damage, and cross-hatched shear band patterns. It appears that histological
damage increases with age 42,43 and is more pronounced in women. 43,44 These correlations have
fueled a large body of research attempting to determine a relationship between mechanical property
degradations and microdamage. True cause-and-effect relationships have not been established and
have been controversial. The ability to detect microdamage at a high enough resolution, as well as
to quantify it unambiguously, has been proposed as a confounding factor.
Damage may have direct biological consequences since the underlying cells will undergo struc-
tural damage as the surrounding bone matrix permanently deforms and sustains microdamage. This
cellular damage may induce a biological response, perhaps prompting the bone cells to repair the
subtle matrix damage. This is an important point when interpreting fatigue or creep properties
because it should be realized that no biological healing can occur during in vitro experiments. Thus