Page 145 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 145
124 MEDICAL DEVICE DESIGN
Pump
Chopper
IR
IR
detector
lamp
Sample
Reference
LED Control
LED
detector circuit
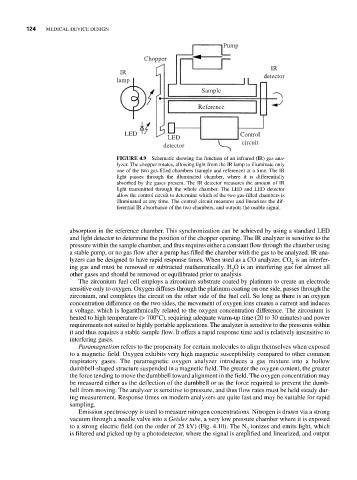
FIGURE 4.9 Schematic showing the function of an infrared (IR) gas ana-
lyzer. The chopper rotates, allowing light from the IR lamp to illuminate only
one of the two gas-filled chambers (sample and reference) at a time. The IR
light passes through the illuminated chamber, where it is differentially
absorbed by the gases present. The IR detector measures the amount of IR
light transmitted through the whole chamber. The LED and LED detector
allow the control circuit to determine which of the two gas-filled chambers is
illuminated at any time. The control circuit measures and linearizes the dif-
ferential IR absorbance of the two chambers, and outputs the usable signal.
absorption in the reference chamber. This synchronization can be achieved by using a standard LED
and light detector to determine the position of the chopper opening. The IR analyzer is sensitive to the
pressure within the sample chamber, and thus requires either a constant flow through the chamber using
a stable pump, or no gas flow after a pump has filled the chamber with the gas to be analyzed. IR ana-
lyzers can be designed to have rapid response times. When used as a CO analyzer, CO is an interfer-
2
ing gas and must be removed or subtracted mathematically. H O is an interfering gas for almost all
2
other gases and should be removed or equilibrated prior to analysis.
The zirconium fuel cell employs a zirconium substrate coated by platinum to create an electrode
sensitive only to oxygen. Oxygen diffuses through the platinum coating on one side, passes through the
zirconium, and completes the circuit on the other side of the fuel cell. So long as there is an oxygen
concentration difference on the two sides, the movement of oxygen ions creates a current and induces
a voltage, which is logarithmically related to the oxygen concentration difference. The zirconium is
heated to high temperature (> 700°C), requiring adequate warm-up time (20 to 30 minutes) and power
requirements not suited to highly portable applications. The analyzer is sensitive to the pressures within
it and thus requires a stable sample flow. It offers a rapid response time and is relatively insensitive to
interfering gases.
Paramagnetism refers to the propensity for certain molecules to align themselves when exposed
to a magnetic field. Oxygen exhibits very high magnetic susceptibility compared to other common
respiratory gases. The paramagnetic oxygen analyzer introduces a gas mixture into a hollow
dumbbell-shaped structure suspended in a magnetic field. The greater the oxygen content, the greater
the force tending to move the dumbbell toward alignment in the field. The oxygen concentration may
be measured either as the deflection of the dumbbell or as the force required to prevent the dumb-
bell from moving. The analyzer is sensitive to pressure, and thus flow rates must be held steady dur-
ing measurement. Response times on modern analyzers are quite fast and may be suitable for rapid
sampling.
Emission spectroscopy is used to measure nitrogen concentrations. Nitrogen is drawn via a strong
vacuum through a needle valve into a Geisler tube, a very low pressure chamber where it is exposed
to a strong electric field (on the order of 25 kV) (Fig. 4.10). The N ionizes and emits light, which
2
is filtered and picked up by a photodetector, where the signal is amplified and linearized, and output