Page 94 - Chemical equilibria Volume 4
P. 94
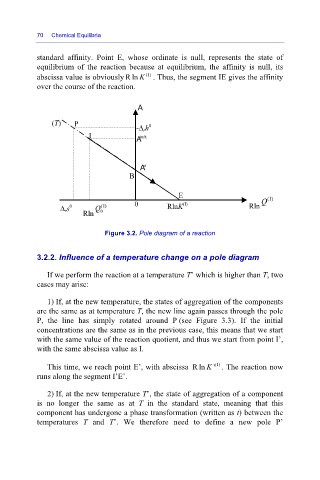
70 Chemical Equilibria
standard affinity. Point E, whose ordinate is null, represents the state of
equilibrium of the reaction because at equilibrium, the affinity is null, its
abscissa value is obviously Rln K (I) . Thus, the segment IE gives the affinity
over the course of the reaction.
A
(T) P -Δ rh 0
I
A init.
A 0
B
E
Δ rs 0 0 RlnK (I) Rln
Rln
Figure 3.2. Pole diagram of a reaction
3.2.2. Influence of a temperature change on a pole diagram
If we perform the reaction at a temperature T’ which is higher than T, two
cases may arise:
1) If, at the new temperature, the states of aggregation of the components
are the same as at temperature T, the new line again passes through the pole
P, the line has simply rotated around P (see Figure 3.3). If the initial
concentrations are the same as in the previous case, this means that we start
with the same value of the reaction quotient, and thus we start from point I’,
with the same abscissa value as I.
(I)
This time, we reach point E’, with abscissa Rln K ' . The reaction now
runs along the segment I’E’.
2) If, at the new temperature T’, the state of aggregation of a component
is no longer the same as at T in the standard state, meaning that this
component has undergone a phase transformation (written as t) between the
temperatures T and T’. We therefore need to define a new pole P’