Page 95 - Chemical equilibria Volume 4
P. 95
Molecular Chemical Equilibria 71
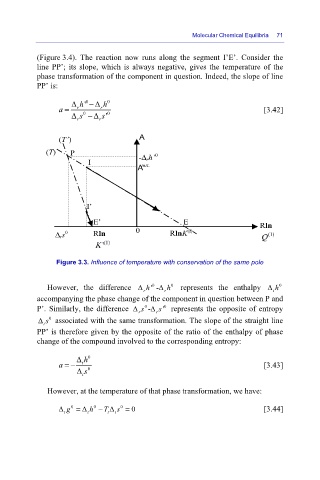
(Figure 3.4). The reaction now runs along the segment I’E’. Consider the
line PP’; its slope, which is always negative, gives the temperature of the
phase transformation of the component in question. Indeed, the slope of line
PP’ is:
Δ ' − Δ h 0
0
h
a = r r [3.42]
Δ r 0 Δ s − r ' s 0
(T’) A
(T) P -Δ rh’ 0
I
A init.
I’
E’ E Rln
Δ rs 0 Rln 0 RlnK (I)
Figure 3.3. Influence of temperature with conservation of the same pole
0
0
0
However, the difference Δ h '-Δ h represents the enthalpy Δ h
r
r
t
accompanying the phase change of the component in question between P and
0
s
P’. Similarly, the difference Δ s 0 -Δ ' represents the opposite of entropy
r r
Δ s associated with the same transformation. The slope of the straight line
0
t
PP’ is therefore given by the opposite of the ratio of the enthalpy of phase
change of the compound involved to the corresponding entropy:
Δ h 0
a =− t [3.43]
Δ s 0
t
However, at the temperature of that phase transformation, we have:
0
Δ t 0 Δ g = t 0 T t Δ h − t s = 0 [3.44]