Page 114 - Corrosion Engineering Principles and Practice
P. 114
88 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 89
30
i = 10 0.8 mA cm –2
o
25
20 Cathodic Branch
Current Density (mA cm –2 ) –10
15
10
5
0
–5
–15
–20 Anodic Branch
–25
–30
0.5 0.4 0.3 0.2 0.1 0 –0.1 –0.2 –0.3 –0.4 –0.5
Overpotential (V)
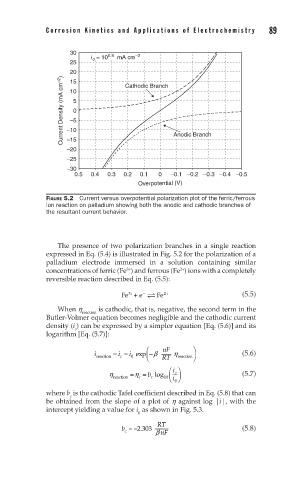
FIGURE 5.2 Current versus overpotential polarization plot of the ferric/ferrous
ion reaction on palladium showing both the anodic and cathodic branches of
the resultant current behavior.
The presence of two polarization branches in a single reaction
expressed in Eq. (5.4) is illustrated in Fig. 5.2 for the polarization of a
palladium electrode immersed in a solution containing similar
3+
2+
concentrations of ferric (Fe ) and ferrous (Fe ) ions with a completely
reversible reaction described in Eq. (5.5):
Fe + e Fe (5.5)
3+
2+
−
When h is cathodic, that is, negative, the second term in the
reaction
Butler-Volmer equation becomes negligible and the cathodic current
density (i ) can be expressed by a simpler equation [Eq. (5.6)] and its
c
logarithm [Eq. (5.7)]:
nF
i reaction = i = i exp −b RT h reaction (5.6)
0
c
i
c
h reaction = h = b c log 10 (5.7)
i
c
0
where b is the cathodic Tafel coefficient described in Eq. (5.8) that can
c
be obtained from the slope of a plot of h against log |i|, with the
intercept yielding a value for i as shown in Fig. 5.3.
0
RT
b = −2.303 b nF (5.8)
c