Page 112 - Corrosion Engineering Principles and Practice
P. 112
86 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 87
function of several factors, including the rate of electron transfer from
a metal to hydrogen ions. In fact, there is a wide variability in this
transfer rate of electrons on various metals and, as a result, the rate of
hydrogen evolution from different metal surfaces can vary greatly.
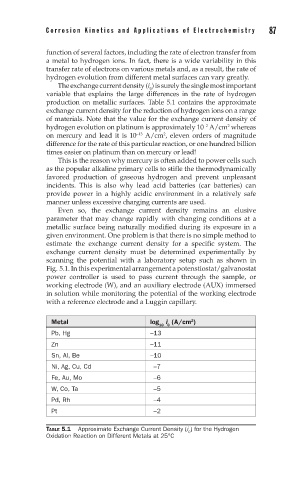
The exchange current density (i ) is surely the single most important
0
variable that explains the large differences in the rate of hydrogen
production on metallic surfaces. Table 5.1 contains the approximate
exchange current density for the reduction of hydrogen ions on a range
of materials. Note that the value for the exchange current density of
hydrogen evolution on platinum is approximately 10 A/cm whereas
−2
2
on mercury and lead it is 10 A/cm , eleven orders of magnitude
−13
2
difference for the rate of this particular reaction, or one hundred billion
times easier on platinum than on mercury or lead!
This is the reason why mercury is often added to power cells such
as the popular alkaline primary cells to stifle the thermodynamically
favored production of gaseous hydrogen and prevent unpleasant
incidents. This is also why lead acid batteries (car batteries) can
provide power in a highly acidic environment in a relatively safe
manner unless excessive charging currents are used.
Even so, the exchange current density remains an elusive
parameter that may change rapidly with changing conditions at a
metallic surface being naturally modified during its exposure in a
given environment. One problem is that there is no simple method to
estimate the exchange current density for a specific system. The
exchange current density must be determined experimentally by
scanning the potential with a laboratory setup such as shown in
Fig. 5.1. In this experimental arrangement a potenstiostat/galvanostat
power controller is used to pass current through the sample, or
working electrode (W), and an auxiliary electrode (AUX) immersed
in solution while monitoring the potential of the working electrode
with a reference electrode and a Luggin capillary.
Metal log i (A/cm )
2
10 0
Pb, Hg –13
Zn –11
Sn, Al, Be –10
Ni, Ag, Cu, Cd –7
Fe, Au, Mo –6
W, Co, Ta –5
Pd, Rh –4
Pt –2
TABLE 5.1 Approximate Exchange Current Density (i ) for the Hydrogen
0
Oxidation Reaction on Different Metals at 25°C