Page 107 - Corrosion Engineering Principles and Practice
P. 107
82 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 83
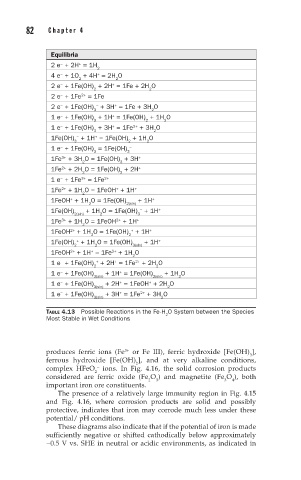
Equilibria
−
2 e + 2H = 1H
+
2
+
4 e + 1O + 4H = 2H O
−
2 2
2 e + 1Fe(OH) + 2H = 1Fe + 2H O
−
+
2 2
2 e + 1Fe = 1Fe
2+
−
−
−
2 e + 1Fe(OH) + 3H = 1Fe + 3H O
+
3 2
−
+
1 e + 1Fe(OH) + 1H = 1Fe(OH) + 1H O
3 2 2
1 e + 1Fe(OH) + 3H = 1Fe + 3H O
+
−
2+
3 2
1Fe(OH) + 1H = 1Fe(OH) + 1H O
+
−
3 2 2
1 e + 1Fe(OH) = 1Fe(OH) −
−
3 3
1Fe + 3H O = 1Fe(OH) + 3H +
3+
2 3
1Fe + 2H O = 1Fe(OH) + 2H +
2+
2 2
−
3+
1 e + 1Fe = 1Fe 2+
2+
+
1Fe + 1H O = 1FeOH + 1H +
2
1FeOH + 1H O = 1Fe(OH) + 1H +
+
2 2(sln)
1Fe(OH) + 1H O = 1Fe(OH) + 1H +
−
2(sln) 2 3
1Fe + 1H O = 1FeOH + 1H +
2+
3+
2
2+
1FeOH + 1H O = 1Fe(OH) + 1H +
+
2 2
1Fe(OH) + 1H O = 1Fe(OH) + 1H +
+
2 2 3(sln)
1FeOH + 1H = 1Fe + 1H O
2+
+
2+
2
2+
+
1 e + 1Fe(OH) + 2H = 1Fe + 2H O
+
−
2 2
1 e + 1Fe(OH) + 1H = 1Fe(OH) + 1H O
−
+
3(sln) 2(sln) 2
1 e + 1Fe(OH) + 2H = 1FeOH + 2H O
+
−
+
3(sln) 2
−
1 e + 1Fe(OH) + 3H = 1Fe + 3H O
2+
+
3(sln) 2
TABLE 4.13 Possible Reactions in the Fe-H O System between the Species
2
Most Stable in Wet Conditions
produces ferric ions (Fe or Fe III), ferric hydroxide [Fe(OH) ],
3+
3
ferrous hydroxide [Fe(OH) ], and at very alkaline conditions,
2
−
complex HFeO ions. In Fig. 4.16, the solid corrosion products
2
considered are ferric oxide (Fe O ) and magnetite (Fe O ), both
4
2
3
3
important iron ore constituents.
The presence of a relatively large immunity region in Fig. 4.15
and Fig. 4.16, where corrosion products are solid and possibly
protective, indicates that iron may corrode much less under these
potential/ pH conditions.
These diagrams also indicate that if the potential of iron is made
sufficiently negative or shifted cathodically below approximately
−0.5 V vs. SHE in neutral or acidic environments, as indicated in