Page 103 - Corrosion Engineering Principles and Practice
P. 103
78 C h a p t e r 4 C o r r o s i o n T h e r m o d y n a m i c s 79
2
1.5
b
1
Potential (V vs. SHE) –0.5 0 a Al AlO 2 –
0.5
3+
–1
–1.5
–2
–2 0 2 4 6 8 10 12 14 16
pH
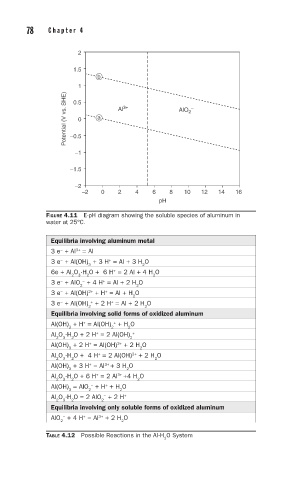
FIGURE 4.11 E-pH diagram showing the soluble species of aluminum in
water at 25°C.
Equilibria involving aluminum metal
−
3 e + Al = Al
3+
3 e + Al(OH) + 3 H = Al + 3 H O
−
+
3 2
6e + Al O ·H O + 6 H = 2 Al + 4 H O
+
2 3 2 2
−
+
−
3 e + AlO + 4 H = Al + 2 H O
2 2
+
2+
3 e + Al(OH) + H = Al + H O
−
2
+
−
+
3 e + Al(OH) + 2 H = Al + 2 H O
2 2
Equilibria involving solid forms of oxidized aluminum
+
+
Al(OH) + H = Al(OH) + H O
3 2 2
Al O ·H O + 2 H = 2 Al(OH) +
+
2 3 2 2
Al(OH) + 2 H = Al(OH) + 2 H O
+
2+
3 2
Al O ·H O + 4 H = 2 Al(OH) + 2 H O
2+
+
2 3 2 2
Al(OH) + 3 H = Al + 3 H O
3+
+
3 2
+
3+
Al O ·H O + 6 H = 2 Al +4 H O
2 3 2 2
Al(OH) = AlO + H + H O
+
−
3 2 2
Al O ·H O = 2 AlO + 2 H +
−
2 3 2 2
Equilibria involving only soluble forms of oxidized aluminum
3+
AlO + 4 H = Al + 2 H O
+
−
2 2
TABLE 4.12 Possible Reactions in the Al-H O System
2