Page 115 - Corrosion Engineering Principles and Practice
P. 115
90 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 91
3
2.5 Anodic Slope Cathodic Slope
Log (|Current Density| (mA cm –2 )) 1.5 Anodic Branch Cathodic Branch
2
1
0.5
0 Log (|io|)
1 0.8 0.6 0.4 0.2 0 –0.2 –0.4 –0.6 –0.8 –1
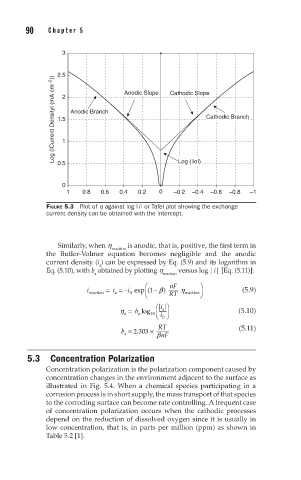
FIGURE 5.3 Plot of h against log | i | or Tafel plot showing the exchange
current density can be obtained with the intercept.
Similarly, when h reaction is anodic, that is, positive, the first term in
the Butler-Volmer equation becomes negligible and the anodic
current density (i ) can be expressed by Eq. (5.9) and its logarithm in
a
Eq. (5.10), with b obtained by plotting h versus log |i| [Eq. (5.11)]:
a reaction
nF
i reaction = i = − i exp (1 b ) RT h reaction (5.9)
−
0
a
a
h = b log 10 i | | (5.10)
a
i
a
0
b = 2.303 × b RT (5.11)
nF
a
5.3 Concentration Polarization
Concentration polarization is the polarization component caused by
concentration changes in the environment adjacent to the surface as
illustrated in Fig. 5.4. When a chemical species participating in a
corrosion process is in short supply, the mass transport of that species
to the corroding surface can become rate controlling. A frequent case
of concentration polarization occurs when the cathodic processes
depend on the reduction of dissolved oxygen since it is usually in
low concentration, that is, in parts per million (ppm) as shown in
Table 5.2 [1].