Page 118 - Corrosion Engineering Principles and Practice
P. 118
92 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 93
D × 10 D × 10
5
5
Cation (cm s ) Anion (cm s )
–1
–1
2
2
H + 9.30 OH – 5.25
Li + 1.03 F – 1.47
Na + 1.33 Cl – 2.03
K + 1.95 NO – 1.90
3
Ca 2+ 0.79 ClO – 1.79
4
Cu 2+ 0.71 SO 2– 1.06
4
Zn 2+ 0.70 CO 2– 0.92
3
O 2.26 HSO – 1.33
2 4
H O 2.44 HCO –1 1.11
2 3
TABLE 5.3 Diffusion Coefficients of Selected Ions at Infinite Dilution in Water
at 25°C
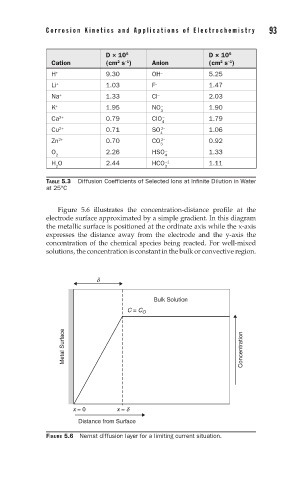
Figure 5.6 illustrates the concentration-distance profile at the
electrode surface approximated by a simple gradient. In this diagram
the metallic surface is positioned at the ordinate axis while the x-axis
expresses the distance away from the electrode and the y-axis the
concentration of the chemical species being reacted. For well-mixed
solutions, the concentration is constant in the bulk or convective region.
d
Bulk Solution
C = C O
Metal Surface Concentration
x = 0 x = d
Distance from Surface
FIGURE 5.6 Nernst diffusion layer for a limiting current situation.