Page 116 - Corrosion Engineering Principles and Practice
P. 116
90 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 91
Temperature Volume Concentration Concentration (M)
(°C) (cm )* (ppm) (µmol L )
3
–1
0 10.2 14.58 455.5
5 8.9 12.72 397.4
10 7.9 11.29 352.8
15 7.0 10.00 312.6
20 6.4 9.15 285.8
25 5.8 8.29 259.0
30 5.3 7.57 236.7
* cm at 0°C per kg of water.
3
TABLE 5.2 Solubility of Oxygen in Air-Saturated Water
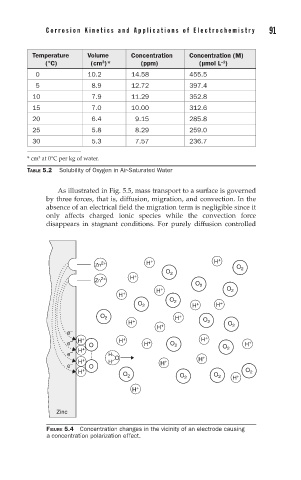
As illustrated in Fig. 5.5, mass transport to a surface is governed
by three forces, that is, diffusion, migration, and convection. In the
absence of an electrical field the migration term is negligible since it
only affects charged ionic species while the convection force
disappears in stagnant conditions. For purely diffusion controlled
Zn 2+ H + H + O
O 2 2
+
Zn 2+ H
O 2
H + O 2
H +
O 2
H
O 2 + H +
O 2 H +
H + H + O 2 O 2
e – +
e – H + O H + H + O 2 H O 2 H +
e – H + H +
H + O H H + H
e – O
H + O 2 O 2 O 2 H + O 2
H +
Zinc
FIGURE 5.4 Concentration changes in the vicinity of an electrode causing
a concentration polarization effect.