Page 120 - Corrosion Engineering Principles and Practice
P. 120
94 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 95
q
Water (W cm)
Pure water 20,000,000
Distilled water 500,000
Rain water 20,000
Tap water 1000–5000
River water (brackish) 200
Sea-water (coastal) 30
Sea-water (open sea) 20–25
TABLE 5.4 Resistivity of Some Typical Waters
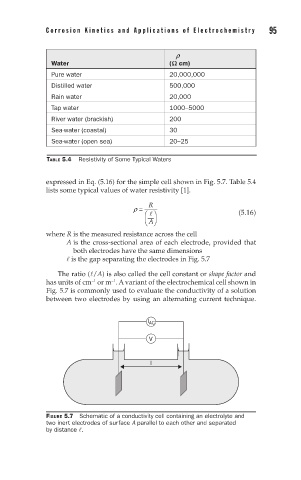
expressed in Eq. (5.16) for the simple cell shown in Fig. 5.7. Table 5.4
lists some typical values of water resistivity [1].
R
r =
(5.16)
A
where R is the measured resistance across the cell
A is the cross-sectional area of each electrode, provided that
both electrodes have the same dimensions
is the gap separating the electrodes in Fig. 5.7
The ratio (/A) is also called the cell constant or shape factor and
has units of cm or m . A variant of the electrochemical cell shown in
−1
−1
Fig. 5.7 is commonly used to evaluate the conductivity of a solution
between two electrodes by using an alternating current technique.
I AC
V
l
FIGURE 5.7 Schematic of a conductivity cell containing an electrolyte and
two inert electrodes of surface A parallel to each other and separated
by distance .