Page 303 - Corrosion Engineering Principles and Practice
P. 303
274 C h a p t e r 8 C o r r o s i o n b y W a t e r 275
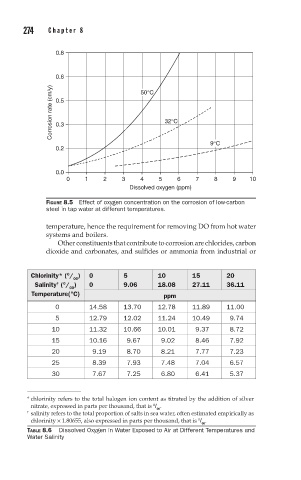
0.8
0.6 50°C
Corrosion rate (cm/y) 0.5 32°C
0.3
9°C
0.2
0.0
0 1 2 3 4 5 6 7 8 9 10
Dissolved oxygen (ppm)
FIGURE 8.5 Effect of oxygen concentration on the corrosion of low-carbon
steel in tap water at different temperatures.
temperature, hence the requirement for removing DO from hot water
systems and boilers.
Other constituents that contribute to corrosion are chlorides, carbon
dioxide and carbonates, and sulfides or ammonia from industrial or
0
Chlorinity* ( / ) 0 5 10 15 20
00
Salinity ( / ) 0 9.06 18.08 27.11 36.11
†
0
00
Temperature(°C) ppm
0 14.58 13.70 12.78 11.89 11.00
5 12.79 12.02 11.24 10.49 9.74
10 11.32 10.66 10.01 9.37 8.72
15 10.16 9.67 9.02 8.46 7.92
20 9.19 8.70 8.21 7.77 7.23
25 8.39 7.93 7.48 7.04 6.57
30 7.67 7.25 6.80 6.41 5.37
* chlorinity refers to the total halogen ion content as titrated by the addition of silver
nitrate, expressed in parts per thousand, that is / .
0
00
† salinity refers to the total proportion of salts in sea water, often estimated empirically as
chlorinity × 1.80655, also expressed in parts per thousand, that is / .
0
00
TABLE 8.6 Dissolved Oxygen in Water Exposed to Air at Different Temperatures and
Water Salinity