Page 304 - Corrosion Engineering Principles and Practice
P. 304
274 C h a p t e r 8 C o r r o s i o n b y W a t e r 275
0.08
0.07 Closed system
Corrosion rate (cm/y) 0.06
0.05
0.04
0.03
0.02
Open system
0.01
0.00
0 20 40 60 80 100 120
Temprature (°C)
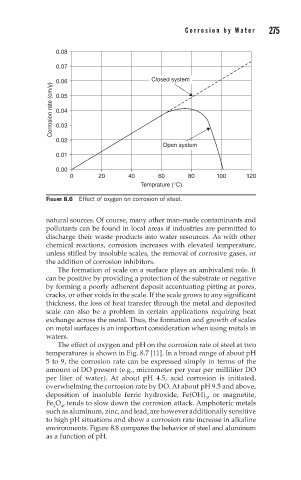
FIGURE 8.6 Effect of oxygen on corrosion of steel.
natural sources. Of course, many other man-made contaminants and
pollutants can be found in local areas if industries are permitted to
discharge their waste products into water resources. As with other
chemical reactions, corrosion increases with elevated temperature,
unless stifled by insoluble scales, the removal of corrosive gases, or
the addition of corrosion inhibitors.
The formation of scale on a surface plays an ambivalent role. It
can be positive by providing a protection of the substrate or negative
by forming a poorly adherent deposit accentuating pitting at pores,
cracks, or other voids in the scale. If the scale grows to any significant
thickness, the loss of heat transfer through the metal and deposited
scale can also be a problem in certain applications requiring heat
exchange across the metal. Thus, the formation and growth of scales
on metal surfaces is an important consideration when using metals in
waters.
The effect of oxygen and pH on the corrosion rate of steel at two
temperatures is shown in Fig. 8.7 [11]. In a broad range of about pH
5 to 9, the corrosion rate can be expressed simply in terms of the
amount of DO present (e.g., micrometer per year per milliliter DO
per liter of water). At about pH 4.5, acid corrosion is initiated,
overwhelming the corrosion rate by DO. At about pH 9.5 and above,
deposition of insoluble ferric hydroxide, Fe(OH) , or magnetite,
3
Fe O , tends to slow down the corrosion attack. Amphoteric metals
4
3
such as aluminum, zinc, and lead, are however additionally sensitive
to high pH situations and show a corrosion rate increase in alkaline
environments. Figure 8.8 compares the behavior of steel and aluminum
as a function of pH.