Page 307 - Corrosion Engineering Principles and Practice
P. 307
278 C h a p t e r 8 C o r r o s i o n b y W a t e r 279
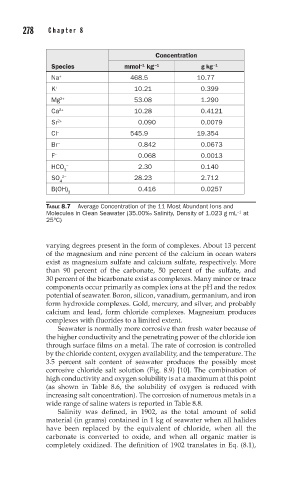
Concentration
Species mmol kg -1 g kg -1
-1
Na + 468.5 10.77
K + 10.21 0.399
Mg 2+ 53.08 1.290
Ca 2+ 10.28 0.4121
Sr 2+ 0.090 0.0079
Cl − 545.9 19.354
Br − 0.842 0.0673
F − 0.068 0.0013
HCO − 2.30 0.140
3
SO 2− 28.23 2.712
4
B(OH) 0.416 0.0257
3
TABLE 8.7 Average Concentration of the 11 Most Abundant Ions and
Molecules in Clean Seawater (35.00‰ Salinity, Density of 1.023 g mL at
−1
25°C)
varying degrees present in the form of complexes. About 13 percent
of the magnesium and nine percent of the calcium in ocean waters
exist as magnesium sulfate and calcium sulfate, respectively. More
than 90 percent of the carbonate, 50 percent of the sulfate, and
30 percent of the bicarbonate exist as complexes. Many minor or trace
components occur primarily as complex ions at the pH and the redox
potential of seawater. Boron, silicon, vanadium, germanium, and iron
form hydroxide complexes. Gold, mercury, and silver, and probably
calcium and lead, form chloride complexes. Magnesium produces
complexes with fluorides to a limited extent.
Seawater is normally more corrosive than fresh water because of
the higher conductivity and the penetrating power of the chloride ion
through surface films on a metal. The rate of corrosion is controlled
by the chloride content, oxygen availability, and the temperature. The
3.5 percent salt content of seawater produces the possibly most
corrosive chloride salt solution (Fig. 8.9) [10]. The combination of
high conductivity and oxygen solubility is at a maximum at this point
(as shown in Table 8.6, the solubility of oxygen is reduced with
increasing salt concentration). The corrosion of numerous metals in a
wide range of saline waters is reported in Table 8.8.
Salinity was defined, in 1902, as the total amount of solid
material (in grams) contained in 1 kg of seawater when all halides
have been replaced by the equivalent of chloride, when all the
carbonate is converted to oxide, and when all organic matter is
completely oxidized. The definition of 1902 translates in Eq. (8.1),