Page 211 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 211
P1: GTY Final pages
Encyclopedia of Physical Science and Technology EN017G-116 August 2, 2001 18:14
Vitamins and Coenzymes 519
per day of retinol or naturally occurring retinol esters is
a safe limit. Amounts of vitamin A are often given in in-
ternational units (IU). One IU is provided by 0.3 µg of
all-trans retinol.
Deficiency of vitamin K is rare in adults but more
frequent in breast-fed infants. The characteristic symptom
of slow blood clotting may also arise, rarely, because of
a hereditary lack of vitamin K-dependent processing of
blood clotting proteins. The exact functions of vitamin
E have been hard to define, but a deficiency can cause
neurological and reproductive problems and muscular
dystrophy in some animals. Although symptoms are rare
in humans, they appear in various hereditary conditions
such as the lack of a liver tocopherol transport protein.
There are eight naturally occurring isomeric forms of
vitamin E (Fig. 3) with differing potencies. The most
active is the natural R, R, R-isomer of α-tocopherol for
which 0.67 mg = 1 IU. At high levels, e.g., 1200 IU per
day, vitamin E may compete with vitamin K and cause
bleeding.
III. CHEMICAL PROPERTIES
AND FUNCTIONS
The major chemical components of cells include the nu-
cleic acids RNA and DNA, polysaccharides (carbohy-
drates), fatty materials (lipids), and many thousands of dif-
ferent proteins. Proteins catalyze most of the metabolism,
the network of chemical reactions by which cells construct
their own substance and by which they obtain and utilize
energy for all life processes. Proteins, whose structure is
dictated by the DNA of the corresponding genes, are pre-
ciselyconstructed.Assubmicroscopicmachinestheyhave
moving parts and apparatus for recognizing and binding to
other molecules, both large and small. Some coenzymes
cooperate with the proteins by carrying electrons, atoms,
or molecular fragments. Others help the proteins to cat-
alyze reactions that are difficult or impossible for the re-
active groups provided by the amino acid side chains of
the proteins.
The B-vitamin-containing coenzymes provide a log-
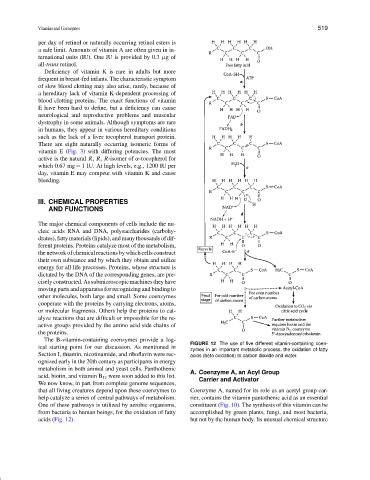
FIGURE 12 The use of five different vitamin-containing coen-
ical starting point for our discussion. As mentioned in
zymes in an important metabolic process, the oxidation of fatty
Section I, thiamin, nicotinamide, and riboflavin were rec- acids (beta oxidation) to carbon dioxide and water.
ognized early in the 20th century as participants in energy
metabolism in both animal and yeast cells. Panthothenic
A. Coenzyme A, an Acyl Group
acid, biotin, and vitamin B 12 were soon added to this list.
Carrier and Activator
We now know, in part from complete genome sequences,
that all living creatures depend upon these coenzymes to Coenzyme A, named for its role as an acetyl group car-
help catalyze a series of central pathways of metabolism. rier, contains the vitamin pantothenic acid as an essential
One of these pathways is utilized by aerobic organisms, constituent (Fig. 10). The synthesis of this vitamin can be
from bacteria to human beings, for the oxidation of fatty accomplished by green plants, fungi, and most bacteria,
acids (Fig. 12). but not by the human body. Its unusual chemical structure