Page 213 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 213
P1: GTY Final pages
Encyclopedia of Physical Science and Technology EN017G-116 August 2, 2001 18:14
Vitamins and Coenzymes 521
−
also facilitates addition of an HO ion at the C3 position
in step c to form an alcohol. The latter is dehydrogenated
+
by NAD in step d.
Another important aspect of FAD chemistry is the abil-
ity to accept a single hydrogen atom (or a single electron
together with a proton) to form a free radical, which we
•
may designate FADH ; the dot indicates the reactive un-
paired electron. This ability allows FAD or FMN to accept
a hydride ion, undergoing a two-electron reduction, then
pass the electrons one at a time to an electron-accepting
metal center in an electron transport chain such as that
found in membranes of the mitochondria. It is at the
ends of these electron transport chains that oxygen (O 2 ),
brought into the human body through the lungs, combines
with four electrons and four protons to form two water
molecules. At the other end of the chain OH groups in
a variety of metabolic intermediates are dehydrogenated
to carbonyl groups by molecules of NAD . The resulting
+
NADH transfers its hydrogen (plus a free H ) to FMN
+
within the mitochondrial chain. These reactions, which
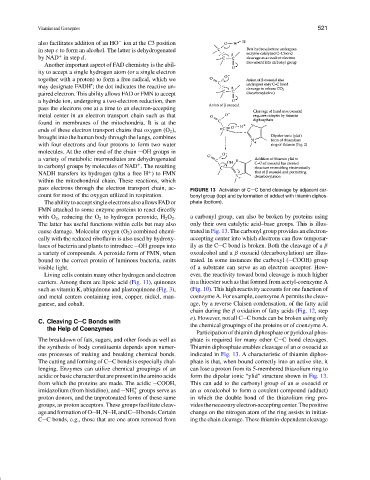
pass electrons through the electron transport chain, ac- FIGURE 13 Activation of C C bond cleavage by adjacent car-
count for most of the oxygen utilized in respiration. bonyl group (top) and by formation of adduct with thiamin diphos-
The ability to accept single electrons also allows FAD or phate (bottom).
FMN attached to some enzyme proteins to react directly
with O 2 , reducing the O 2 to hydrogen peroxide, H 2 O 2 . a carbonyl group, can also be broken by proteins using
The latter has useful functions within cells but may also only their own catalytic acid–base groups. This is illus-
cause damage. Molecular oxygen (O 2 ) combined chemi- trated in Fig. 13. The carbonyl group provides an electron-
cally with the reduced riboflavin is also used by hydroxy- accepting center into which electrons can flow temporar-
lases of bacteria and plants to introduce OH groups into ily as the C C bond is broken. Both the cleavage of a β
a variety of compounds. A peroxide form of FMN, when oxoalcohol and a β oxoacid (decarboxylation) are illus-
bound to the correct protein of luminous bacteria, emits trated. In some instances the carboxyl ( COOH) group
visible light. of a substrate can serve as an electron acceptor. How-
Living cells contain many other hydrogen and electron ever, the reactivity toward bond cleavage is much higher
carriers. Among them are lipoic acid (Fig. 11), quinones in a thioester such as that formed from acetyl-coenzyme A
such as vitamin K, ubiquinone and plastoquinone (Fig. 3), (Fig. 10). This high reactivity accounts for one function of
and metal centers containing iron, copper, nickel, man- coenzyme A. For example, coenzyme A permits the cleav-
ganese, and cobalt. age, by a reverse Claisen condensation, of the fatty acid
chain during the β oxidation of fatty acids (Fig. 12, step
e). However, not all C C bonds can be broken using only
C. Cleaving C C Bonds with
the chemical groupings of the proteins or of coenzyme A.
the Help of Coenzymes
Participation of thiamin diphosphate or pyridoxal phos-
The breakdown of fats, sugars, and other foods as well as phate is required for many other C C bond cleavages.
the synthesis of body constituents depends upon numer- Thiamin diphosphate enables cleavage of an α oxoacid as
ous processes of making and breaking chemical bonds. indicated in Fig. 13. A characteristic of thiamin diphos-
The cutting and forming of C C bonds is especially chal- phate is that, when bound correctly into an active site, it
lenging. Enzymes can utilize chemical groupings of an can lose a proton from its 5-membered thiazolium ring to
acidic or basic character that are present in the amino acids form the dipolar ionic “ylid” structure shown in Fig. 13.
from which the proteins are made. The acidic COOH, This can add to the carbonyl group of an α oxoacid or
imidazolium (from histidine), and NH groups serve as an α oxoalcohol to form a covalent compound (adduct)
+
3
proton donors, and the unprotonated forms of these same in which the double bond of the thiazolium ring pro-
groups, as proton acceptors. These groups facilitate cleav- videsthenecessaryelectron-acceptingcenter.Thepositive
ageandformationofO H,N H,andC Hbonds.Certain change on the nitrogen atom of the ring assists in initiat-
C C bonds, e.g., those that are one atom removed from ing the chain cleavage. These thiamin-dependent cleavage