Page 215 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 215
P1: GTY Final pages
Encyclopedia of Physical Science and Technology EN017G-116 August 2, 2001 18:14
Vitamins and Coenzymes 523
the coenzyme, with a proton bound to the nitrogen atom carrying a different group may replace Y. Protonation of
of its ring, serves as an electron acceptor in much the same the new Schiff base that results yields a new amino acid.
way as does thiamin diphosphate (Fig. 13). The structure Several amino acids are made by plants and microorgan-
resulting from removal of the α-proton of the PLP Schiff isms using this reaction sequence. Returning to the top of
base is variously known as a “quinonoid” or “carbanionic” Fig. 14, notice that cleavage of bond b leads to forma-
intermediate. Depending upon the specificity of the en- tion of CO 2 and decarboxylation of the substrate amino
zyme in whose site it is formed, this intermediate may acid. In this way the amino acid dihydroxyphenylalanine
react in several ways. In a bacterial racemase a proton (dopa) is converted to the neurotransmitter dopamine. The
may be returned to the α-carbon atom from which it was latter can then be hydroxylated and methylated to form the
removed but without stereospecificity, i.e., into either of hormone adrenaline. Histidine is converted by decarboxy-
two positions relative to the other groups surrounding the lation to histamine, a problem compound in allergic reac-
α-carbon. Some racemases are used by bacteria to convert tions, while in the brain, the major excitatory neurotrans-
the stereoisomer known as L-alanine into the less com- mitter is decarboxylated to gamma-aminobutyrate (gaba).
mon “unnatural” D-alanine. The latter is incorporated into This is the major inhibitory transmitter in the central ner-
the bacterial cell wall and helps provide protection to the vous system and the compound that keeps our brains calm
bacteria against attack by hydrolytic enzymes. enough to function. Cleavage of bond c (Fig. 14), when
A second mode of reaction of the quinonoid-carban- R H and Y OH (the amino acid is serine) releases the
ionicintermediateisutilizedbyplantswhichsynthesizean single-carbon compound formaldehyde. This process also
enzyme that acts on the amino acid S-adenosylmethionine requires tetrahydrofolate (Fig. 6). In a converse type of
to form a cyclic three-membered ring compound aminocy- reaction glycine or serine may be condensed with vari-
clopropanecarboxylicacid.Thisisamajorplanthormone. ous carbonyl compounds to initiate new biosynthetic path-
In a third type of reaction a proton is added back to the ways. These are often coupled to decarboxylation, which
coenzyme itself (see Fig. 14) to form what is called a helps to drive the sequence in the biosynthetic direction.
ketimine (not illustrated). This is a Schiff base of pyridox- One of these yields the red heme pigment of blood.
amine phosphate (PMP, Fig. 5) with an α-oxoacid and is an A third coenzyme that is involved in C C bond
essential intermediate compound in the important process cleavage and formation is the vitamin B 12 derivative
of transamination (Fig. 14). This process is utilized by all 5 -deoxyadenosylcobalamin (Fig. 7). In this compound
living organisms both in the synthesis of amino acids and the cobalt–carbon bond is easily cleaved to form a free
in the breakdown of excesses of amino acids. The human radical which, in turn, facilitates C C bond cleavage in
body forms several amino acids via transamination. As the substrate. The details, which are still under study, have
shown in Fig. 15, this is a reversible sequence involving a beenomitted,butFig.16showsageneralreactioninwhich
cyclic interconversion of PLP and PMP in reaction steps
of the type illustrated in Fig. 14.
Yet another reaction for the ketimine illustrated in
Fig. 14 is the elimination of a substituent (labeled Y in this
drawing) with formation of a double bond. The product
of this elimination sometimes decomposes, with loss of
nitrogen as ammonia (NH 3 ), but in other cases a molecule
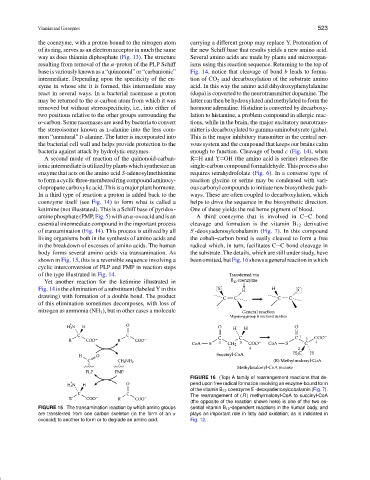
FIGURE 16 (Top) A family of rearrangement reactions that de-
pend upon free radical formation involving an enzyme-bound form
of the vitamin B 12 coenzyme 5 -deoxyadenosylcobalamin (Fig. 7).
The rearrangement of (R ) methylmalonyl-CoA to succinyl-CoA
(the opposite of the reaction shown here) is one of the two es-
FIGURE 15 The transamination reaction by which amino groups sential vitamin B 12 -dependent reactions in the human body, and
are transferred from one carbon skeleton (in the form of an α plays an important role in fatty acid oxidation, as is indicated in
oxoacid) to another to form or to degrade an amino acid. Fig. 12.