Page 218 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 218
P1: GOJ/GOI/LCC/HAR P2: GOJ Final Pages
Encyclopedia of Physical Science and Technology en012K-946 July 26, 2001 11:14
Polymers, Photoresponsive 727
poration of styrene into the resist improves the dry
etching characteristics of the polymers, these materi-
als are often favored over aliphatic-based resists. Spe-
cific examples of halogenated styrene-based resists are
chlorinated 23 or chloromethylated 24 polystyrene and
poly(chloromethylstyrene). 25,26 In addition to being sensi-
tive electron-beam resists, the latter also have sensitivity to
deep-UV 27 and X-ray 28 exposure. In one case, the halo-
genated material chlorostyrene was copolymerized with
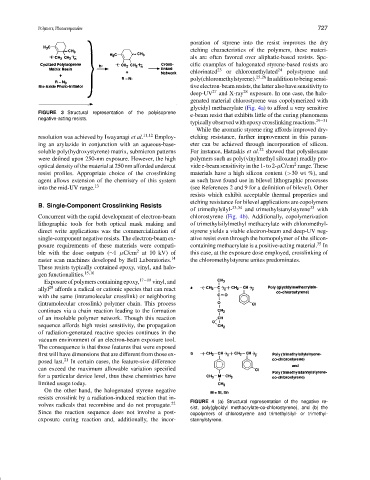
glycidyl methacrylate (Fig. 4a) to afford a very sensitive
FIGURE 3 Structural representation of the polyisoprene
e-beam resist that exhibits little of the curing phenomena
negative-acting resists. 29–31
typically observed with epoxy crosslinking reactions.
While the aromatic styrene ring affords improved dry-
resolution was achieved by Iwayanagi et al. 11,12 Employ- etching resistance, further improvement in this param-
ing an arylazide in conjunction with an aqueous-base- eter can be achieved through incorporation of silicon.
soluble poly(hydroxystyrene) matrix, submicron patterns For instance, Hatzakis et al. 32 showed that polysiloxane
were defined upon 250-nm exposure. However, the high polymers such as poly(vinylmethyl siloxane) readily pro-
2
optical density of the material at 250 nm afforded undercut vide e-beam sensitivity in the 1- to 2-µC/cm range. These
resist profiles. Appropriate choice of the crosslinking materials have a high silicon content (>30 wt %), and
agent allows extension of the chemistry of this system as such have found use in bilevel lithographic processes
into the mid-UV range. 13 (see References 2 and 9 for a definition of bilevel). Other
resists which exhibit acceptable thermal properties and
etching resistance for bilevel applications are copolymers
B. Single-Component Crosslinking Resists 33,34 33
of trimethylsilyl- and trimethylstanylstyrene with
Concurrent with the rapid development of electron-beam chlorostyrene (Fig. 4b). Additionally, copolymerization
lithographic tools for both optical mask making and of trimethylsilylmethyl methacrylate with chloromethyl-
direct write applications was the commercialization of styrene yields a viable electron-beam and deep-UV neg-
single-component negative resists. The electron-beam ex- ative resist even through the homopolymer of the silicon-
35
posure requirements of these materials were compati- containing methacrylate is a positive-acting material. In
2
ble with the dose outputs (∼1 µC/cm at 10 kV) of this case, at the exposure dose employed, crosslinking of
raster scan machines developed by Bell Laboratories. 14 the chloromethylstyrene unites predominates.
These resists typically contained epoxy, vinyl, and halo-
gen functionalities. 15,16
Exposureofpolymerscontainingepoxy, 17−19 vinyl,and
allyl 20 affords a radical or cationic species that can react
with the same (intramolecular crosslink) or neighboring
(intramolecular crosslink) polymer chain. This process
continues via a chain reaction leading to the formation
of an insoluble polymer network. Though this reaction
sequence affords high resist sensitivity, the propagation
of radiation-generated reactive species continues in the
vacuum environment of an electron-beam exposure tool.
The consequence is that those features that were exposed
first will have dimensions that are different from those ex-
posed last. 21 In certain cases, the feature-size difference
can exceed the maximum allowable variation specified
for a particular device level, thus these chemistries have
limited usage today.
On the other hand, the halogenated styrene negative
resists crosslink by a radiation-induced reaction that in- FIGURE 4 (a) Structural representation of the negative re-
volves radicals that recombine and do not propagate. 22
sist, poly(glycidyl methacrylate-co-chlorostyrene), and (b) the
Since the reaction sequence does not involve a post- copolymers of chlorostyrene and trimethylsilyl- or trimethyl-
exposure curing reaction and, additionally, the incor- stannylstyrene.