Page 223 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 223
P1: GOJ/GOI/LCC/HAR P2: GOJ Final Pages
Encyclopedia of Physical Science and Technology en012K-946 July 26, 2001 11:14
732 Polymers, Photoresponsive
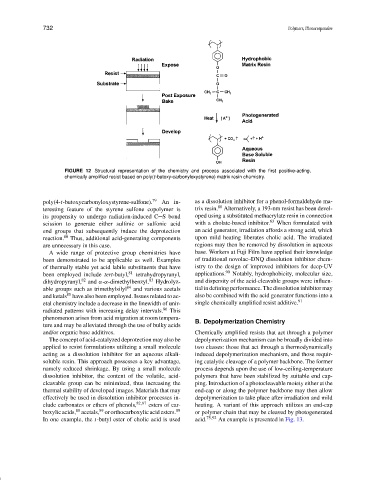
FIGURE 12 Structural representation of the chemistry and process associated with the first positive-acting,
chemically amplified resist based on poly(t-butoxy-carbonyloxystyrene) matrix resin chemistry.
poly(4-t-butoxycarbonyloxystyrene-sulfone). 79 An in- as a dissolution inhibitor for a phenol-formaldehyde ma-
88
teresting feature of the styrene sulfone copolymer is trix resin. Alternatively, a 193-nm resist has been devel-
its propensity to undergo radiation-induced C S bond oped using a substituted methacrylate resin in connection
scission to generate either sulfinic or sulfonic acid with a cholate-based inhibitor. 63 When formulated with
end groups that subsequently induce the deprotection an acid generator, irradiation affords a strong acid, which
reaction. 80 Thus, additional acid-generating components upon mild heating liberates cholic acid. The irradiated
are unnecessary in this case. regions may then be removed by dissolution in aqueous
A wide range of protective group chemistries have base. Workers at Fuji Film have applied their knowledge
been demonstrated to be applicable as well. Examples of traditional novolac–DNQ dissolution inhibitor chem-
of thermally stable yet acid labile substituents that have istry to the design of improved inhibitors for deep-UV
been employed include tert-butyl, 81 tetrahydropyranyl, applications. 90 Notably, hydrophobicity, molecular size,
dihydropyranyl, 82 and α-α-dimethylbenzyl. 83 Hydrolyz- and dispersity of the acid-cleavable groups were influen-
able groups such as trimethylsilyl 84 and various acetals tial in defining performance. The dissolution inhibitor may
85
and ketals have also been employed. Issues related to ac- also be combined with the acid generator functions into a
etal chemistry include a decrease in the linewidth of unir- single chemically amplified resist additive. 91
radiated patterns with increasing delay intervals. 86 This
phenomenon arises from acid migration at room tempera-
B. Depolymerization Chemistry
ture and may be alleviated through the use of bulky acids
and/or organic base additives. Chemically amplified resists that act through a polymer
The concept of acid-catalyzed deprotection may also be depolymerization mechanism can be broadly divided into
applied to resist formulations utilizing a small molecule two classes: those that act through a thermodynamically
acting as a dissolution inhibitor for an aqueous alkali- induced depolymerization mechanism, and those requir-
soluble resin. This approach possesses a key advantage, ing catalytic cleavage of a polymer backbone. The former
namely reduced shrinkage. By using a small molecule process depends upon the use of low-ceiling-temperature
dissolution inhibitor, the content of the volatile, acid- polymers that have been stabilized by suitable end cap-
cleavable group can be minimized, thus increasing the ping. Introduction of a photocleavable moiety either at the
thermal stability of developed images. Materials that may end-cap or along the polymer backbone may then allow
effectively be used in dissolution inhibitor processes in- depolymerization to take place after irradiation and mild
clude carbonates or ethers of phenols, 82,87 esters of car- heating. A variant of this approach utilizes an end-cap
89
88
boxylic acids, acetals, or orthocarboxylic acid esters. 89 or polymer chain that may be cleaved by photogenerated
In one example, the t-butyl ester of cholic acid is used acid. 75,92 An example is presented in Fig. 13.