Page 221 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 221
P1: GOJ/GOI/LCC/HAR P2: GOJ Final Pages
Encyclopedia of Physical Science and Technology en012K-946 July 26, 2001 11:14
730 Polymers, Photoresponsive
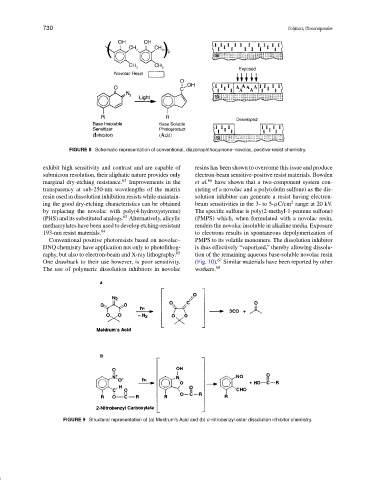
FIGURE 8 Schematic representation of conventional, diazonaphthoquinone–novolac, positive-resist chemistry.
exhibit high sensitivity and contrast and are capable of resins has been shown to overcome this issue and produce
submicron resolution, their aliphatic nature provides only electron-beam sensitive-positive resist materials. Bowden
marginal dry-etching resistance. 62 Improvements in the et al. 66 have shown that a two-component system con-
transparency at sub-250-nm wavelengths of the matrix sisting of a novolac and a poly(olefin sulfone) as the dis-
resin used in dissolution inhibition resists while maintain- solution inhibitor can generate a resist having electron-
2
ing the good dry-etching characteristics can be obtained beam sensitivities in the 3- to 5-µC/cm range at 20 kV.
by replacing the novolac with poly(4-hydroxystyrene) The specific sulfone is poly(2-methyl-1-pentene sulfone)
63
(PHS) and its substituted analogs. Alternatively, alicylic (PMPS) which, when formulated with a novolac resin,
methacrylates have been used to develop etching-resistant renders the novolac insoluble in alkaline media. Exposure
193-nm resist materials. 64 to electrons results in spontaneous depolymerization of
Conventional positive photoresists based on novolac– PMPS to its volatile monomers. The dissolution inhibitor
DNQ chemistry have application not only to photolithog- is thus effectively “vaporized,” thereby allowing dissolu-
raphy, but also to electron-beam and X-ray lithography. 65 tion of the remaining aqueous base-soluble novolac resin
67
One drawback to their use however, is poor sensitivity. (Fig. 10). Similar materials have been reported by other
The use of polymeric dissolution inhibitors in novolac workers. 68
FIGURE 9 Structural representation of (a) Meldrum’s Acid and (b) o-nitrobenzyl ester dissolution inhibitor chemistry.