Page 346 - Engineering Plastics Handbook
P. 346
Polyarylethersulfones (PAES) 303
It is also attributed to the sulfone group. The strong dipole interactions
and restricted rotation of the aromatic units relative to the other groups
contribute to the rigidity of the molecule. Other connecting groups can
result in either an increase or a decrease in the rigidity of the polymer chain
and T , depending on the polarity and the conformational freedom
g
imparted by those groups.
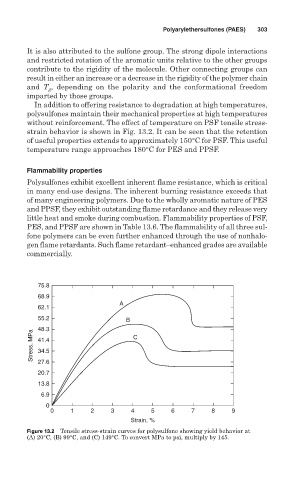
In addition to offering resistance to degradation at high temperatures,
polysulfones maintain their mechanical properties at high temperatures
without reinforcement. The effect of temperature on PSF tensile stress-
strain behavior is shown in Fig. 13.2. It can be seen that the retention
of useful properties extends to approximately 150°C for PSF. This useful
temperature range approaches 180°C for PES and PPSF.
Flammability properties
Polysulfones exhibit excellent inherent flame resistance, which is critical
in many end-use designs. The inherent burning resistance exceeds that
of many engineering polymers. Due to the wholly aromatic nature of PES
and PPSF, they exhibit outstanding flame retardance and they release very
little heat and smoke during combustion. Flammability properties of PSF,
PES, and PPSF are shown in Table 13.6. The flammability of all three sul-
fone polymers can be even further enhanced through the use of nonhalo-
gen flame retardants. Such flame retardant–enhanced grades are available
commercially.
75.8
68.9
A
62.1
55.2 B
48.3
Stress, MPa 41.4 C
34.5
27.6
20.7
13.8
6.9
0
0 1 2 3 4 5 6 7 8 9
Strain, %
Figure 13.2 Tensile stress-strain curves for polysulfone showing yield behavior at
(A) 20°C, (B) 99°C, and (C) 149°C. To convert MPa to psi, multiply by 145.