Page 157 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 157
Methods for Structural and Chemical Characterization of Nanomaterials 143
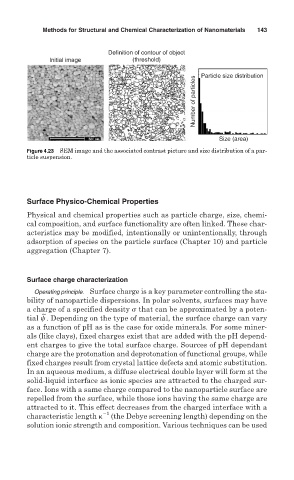
Definition of contour of object
Initial image (threshold) Particle size distribution
Number of particles
Size (area)
Figure 4.23 SEM image and the associated contrast picture and size distribution of a par-
ticle suspension.
Surface Physico-Chemical Properties
Physical and chemical properties such as particle charge, size, chemi-
cal composition, and surface functionality are often linked. These char-
acteristics may be modified, intentionally or unintentionally, through
adsorption of species on the particle surface (Chapter 10) and particle
aggregation (Chapter 7).
Surface charge characterization
Operating principle. Surface charge is a key parameter controlling the sta-
bility of nanoparticle dispersions. In polar solvents, surfaces may have
a charge of a specified density that can be approximated by a poten-
~
tial . Depending on the type of material, the surface charge can vary
as a function of pH as is the case for oxide minerals. For some miner-
als (like clays), fixed charges exist that are added with the pH depend-
ent charges to give the total surface charge. Sources of pH dependant
charge are the protonation and deprotonation of functional groups, while
fixed charges result from crystal lattice defects and atomic substitution.
In an aqueous medium, a diffuse electrical double layer will form at the
solid-liquid interface as ionic species are attracted to the charged sur-
face. Ions with a same charge compared to the nanoparticle surface are
repelled from the surface, while those ions having the same charge are
attracted to it. This effect decreases from the charged interface with a
characteristic length 1 (the Debye screening length) depending on the
solution ionic strength and composition. Various techniques can be used