Page 273 - Fundamentals of Gas Shale Reservoirs
P. 273
DIFFUSION IN BULK KEROGEN 253
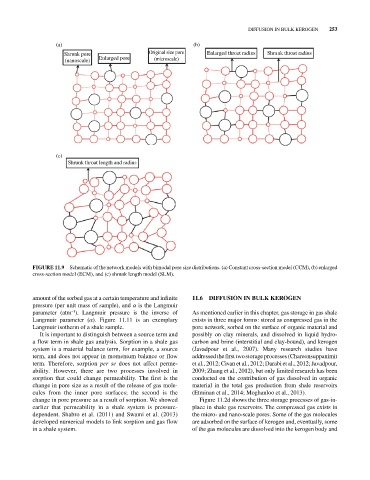
(a) (b)
Shrunk pore Original size pore Enlarged throat radius Shrunk throat radius
(nanoscale) Enlarged pore (microscale)
(c)
Shrunk throat length and radius
FIGURE 11.9 Schematic of the network models with bimodal pore size distributions. (a) Constant cross‐section model (CCM), (b) enlarged
cross‐section model (ECM), and (c) shrunk length model (SLM).
amount of the sorbed gas at a certain temperature and infinite 11.6 DIFFUSION IN bULK KEROGEN
pressure (per unit mass of sample), and α is the Langmuir
parameter (atm ). Langmuir pressure is the inverse of As mentioned earlier in this chapter, gas storage in gas shale
−1
Langmuir parameter (α). Figure 11.11 is an exemplary exists in three major forms: stored as compressed gas in the
Langmuir isotherm of a shale sample. pore network, sorbed on the surface of organic material and
It is important to distinguish between a source term and possibly on clay minerals, and dissolved in liquid hydro-
a flow term in shale gas analysis. Sorption in a shale gas carbon and brine (interstitial and clay‐bound), and kerogen
system is a material balance term, for example, a source (Javadpour et al., 2007). Many research studies have
term, and does not appear in momentum balance or flow addressed the first two storage processes (Chareonsuppanimit
term. Therefore, sorption per se does not affect perme- et al., 2012; Civan et al., 2012; Darabi et al., 2012; Javadpour,
ability. However, there are two processes involved in 2009; Zhang et al., 2012), but only limited research has been
sorption that could change permeability. The first is the conducted on the contribution of gas dissolved in organic
change in pore size as a result of the release of gas mole- material in the total gas production from shale reservoirs
cules from the inner pore surfaces; the second is the (Etminan et al., 2014; Moghanloo et al., 2013).
change in pore pressure as a result of sorption. We showed Figure 11.2d shows the three storage processes of gas‐in‐
earlier that permeability in a shale system is pressure‐ place in shale gas reservoirs. The compressed gas exists in
dependent. Shabro et al. (2011) and Swami et al. (2013) the micro‐ and nano‐scale pores. Some of the gas molecules
developed numerical models to link sorption and gas flow are adsorbed on the surface of kerogen and, eventually, some
in a shale system. of the gas molecules are dissolved into the kerogen body and