Page 350 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 350
Flocculation 305
10,000 10,000
Viscous dissipation Inertial advection
subrange subrange
Ferric floc
5
•
1,000 d(floc-ferric) =7.1 10 μm/G 2 Sludge age = 3.2 days 0.17
d(floc) (μm) d(floc-alum) =1.2 10 μm/G λ(floc) max (μm) Sludge age = 11.9 d
λ(floc-3.2 days)=6000/G
Alum floc
4
•
100 λ(floc-11.9 days)=6000/G 0.37
Kolmogorov’s microscale Sludge age = 9.9 days
λ(floc-9.9 days)=6000/G 0.35
10 1,000
10 100 1000 10 100
–1
–1
(a) G (s ) (b) G (s )
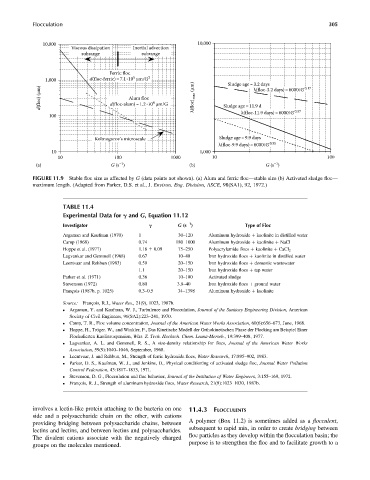
FIGURE 11.9 Stable floc size as affected by G (data points not shown). (a) Alum and ferric floc—stable size (b) Activated sludge floc—
maximum length. (Adapted from Parker, D.S. et al., J. Environ. Eng. Division, ASCE, 98(SA1), 92, 1972.)
TABLE 11.4
Experimental Data for g and G, Equation 11.12
1
Investigator g G (s ) Type of Floc
Argaman and Kaufman (1970) 1 30–120 Aluminum hydroxide þ kaolinite in distilled water
Camp (1968) 0.74 180–1000 Aluminum hydroxide þ kaolinite þ NaCl
Hoppe et al. (1977) 1.18 0.09 75–250 Polyacrylamide flocs þ kaolinite þ CaCl 2
Lagvankar and Gemmell (1968) 0.67 10–40 Iron hydroxide flocs þ kaolinite in distilled water
Leentvaar and Rehbun (1983) 0.59 20–150 Iron hydroxide flocs þ domestic wastewater
1.1 20–150 Iron hydroxide flocs þ tap water
Parker et al. (1971) 0.36 10–100 Activated sludge
Stevenson (1972) 0.80 3.8–40 Iron hydroxide flocs þ ground water
François (1987b, p. 1025) 0.3–0.5 34–1398 Aluminum hydroxide þ kaolinite
Source: François, R.J., Water Res., 21(9), 1023, 1987b.
. Argaman, Y. and Kaufman, W. J., Turbulence and Flocculation, Journal of the Sanitary Engineering Division, American
Society of Civil Engineers, 96(SA2):223–241, 1970.
. Camp, T. R., Floc volume concentration, Journal of the American Water Works Association, 60(6):656–673, June, 1968.
. Hoppe, H., Tröger. W., and Winkler, F., Das Kinetische Modell der Orthokinetischen Phase der Flocking am Beispiel Einer
Flockulierten Kaolinsuspension, Wiss. Z. Tech. Hochsch. Chem. Leuna-Merseb., 19:399–408, 1977.
. Lagvankar, A. L. and Gemmell, R. S., A size-density relationship for flocs, Journal of the American Water Works
Association, 59(9):1040–1046, September, 1968.
. Leentvaar, J. and Rehbun, M., Strength of ferric hydroxide flocs, Water Research, 17:895–902, 1983.
. Parker, D. S., Kaufman, W. J., and Jenkins, D., Physical conditioning of activated sludge floc, Journal Water Pollution
Control Federation, 43:1817–1833, 1971.
. Stevenson, D. G., Flocculation and floc behavior, Journal of the Institution of Water Engineers, 3:155–169, 1972.
. François, R. J., Strength of aluminum hydroxide flocs, Water Research, 21(9):1023–1030, 1987b.
involves a lectin-like protein attaching to the bacteria on one 11.4.3 FLOCCULENTS
side and a polysaccharide chain on the other, with cations
A polymer (Box 11.2) is sometimes added as a flocculent,
providing bridging between polysaccharide chains, between
subsequent to rapid mix, in order to create bridging between
lectins and lectins, and between lectins and polysaccharides.
floc particles as they develop within the flocculation basin; the
The divalent cations associate with the negatively charged
purpose is to strengthen the floc and to facilitate growth to a
groups on the molecules mentioned.