Page 346 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 346
Flocculation 301
(a) (b)
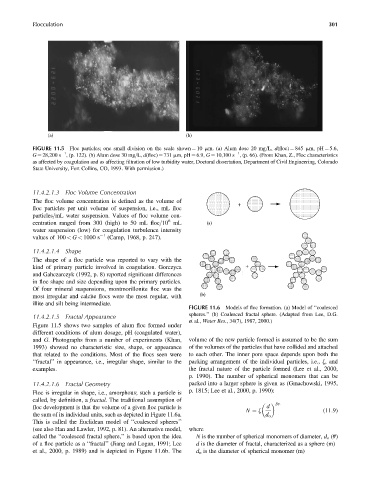
FIGURE 11.5 Floc particles; one small division on the scale shown ¼ 10 mm. (a) Alum dose 20 mg=L, d(floc) ¼ 845 mm, pH ¼ 5.6,
1
1
G ¼ 28,200 s , (p. 122). (b) Alum dose 30 mg=L, d(floc) ¼ 731 mm, pH ¼ 6.9, G ¼ 10,100 s , (p. 66). (From Khan, Z., Floc characteristics
as affected by coagulation and as affecting filtration of low turbidity water, Doctoral dissertation, Department of Civil Engineering, Colorado
State University, Fort Collins, CO, 1993. With permission.)
11.4.2.1.3 Floc Volume Concentration
The floc volume concentration is defined as the volume of
+
floc particles per unit volume of suspension, i.e., mL floc
particles=mL water suspension. Values of floc volume con-
6
centration ranged from 300 (high) to 50 mL floc=10 mL (a)
water suspension (low) for coagulation turbulence intensity
values of 100 < G < 1000 s 1 (Camp, 1968, p. 247).
11.4.2.1.4 Shape
The shape of a floc particle was reported to vary with the
kind of primary particle involved in coagulation. Gorczyca +
and Gahczarczyk (1992, p. 8) reported significant differences
in floc shape and size depending upon the primary particles.
Of four mineral suspensions, montmorillonite floc was the
most irregular and calcite flocs were the most regular, with (b)
illite and silt being intermediate.
FIGURE 11.6 Models of floc formation. (a) Model of ‘‘coalesced
11.4.2.1.5 Fractal Appearance spheres.’’ (b) Coalesced fractal sphere. (Adapted from Lee, D.G.
et al., Water Res., 34(7), 1987, 2000.)
Figure 11.5 shows two samples of alum floc formed under
different conditions of alum dosage, pH (coagulated water),
and G. Photographs from a number of experiments (Khan, volume of the new particle formed is assumed to be the sum
1993) showed no characteristic size, shape, or appearance of the volumes of the particles that have collided and attached
that related to the conditions. Most of the flocs seen were to each other. The inner pore space depends upon both the
‘‘fractal’’ in appearance, i.e., irregular shape, similar to the packing arrangement of the individual particles, i.e., z, and
examples. the fractal nature of the particle formed (Lee et al., 2000,
p. 1990). The number of spherical monomers that can be
11.4.2.1.6 Fractal Geometry packed into a larger sphere is given as (Gmachowski, 1995,
p. 1815; Lee et al., 2000, p. 1990):
Floc is irregular in shape, i.e., amorphous; such a particle is
called, by definition, a fractal. The traditional assumption of
D F
floc development is that the volume of a given floc particle is d
N ¼ z (11:9)
the sum of its individual units, such as depicted in Figure 11.6a. d o
This is called the Euclidean model of ‘‘coalesced spheres’’
(see also Han and Lawler, 1992, p. 81). An alternative model, where
called the ‘‘coalesced fractal sphere,’’ is based upon the idea N is the number of spherical monomers of diameter, d o (#)
of a floc particle as a ‘‘fractal’’ (Jiang and Logan, 1991; Lee d is the diameter of fractal, characterized as a sphere (m)
et al., 2000, p. 1989) and is depicted in Figure 11.6b. The d o is the diameter of spherical monomer (m)