Page 512 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 512
Adsorption 467
Another relation is that the total number of adsorption sites, phase concentration of adsorbate did not change with time,
X m , equals the sum of unoccupied sites, X, plus occupied sites, i.e., after several days or weeks.
X, i.e., at any time,
15.2.1.2.2 Determining Constants=Linearized Form
X m ¼ X þ X (15:8) Equation 15.11 can be arranged to give a linearized form,
C* 1 1
and at equilibrium, C* (15:12)
¼ þ
X* aX m X m
X m ¼ X* þ X* (15:9)
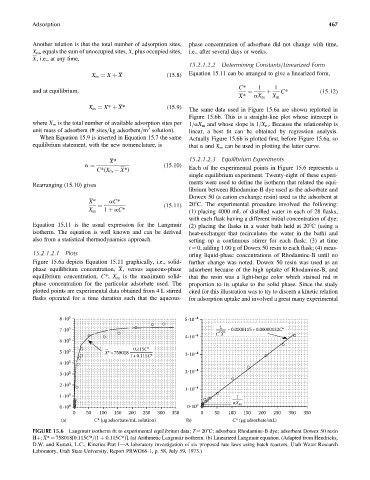
The same data used in Figure 15.6a are shown replotted in
Figure 15.6b. This is a straight-line plot whose intercept is
where X m is the total number of available adsorption sites per 1=aX m and whose slope is 1=X m . Because the relationship is
3
unit mass of adsorbent (# sites=kg adsorbent=m solution). linear, a best fit can be obtained by regression analysis.
When Equation 15.9 is inserted in Equation 15.7 the same Actually Figure 15.6b is plotted first, before Figure 15.6a, so
equilibrium statement, with the new nomenclature, is that a and X m can be used in plotting the latter curve.
X* 15.2.1.2.3 Equilibrium Experiments
(15:10)
a ¼ Each of the experimental points in Figure 15.6 represents a
C*(X m X*)
single equilibrium experiment. Twenty-eight of these experi-
ments were used to define the isotherm that related the equi-
Rearranging (15.10) gives
librium between Rhodamine-B dye used as the adsorbate and
Dowex 50 (a cation exchange resin) used as the adsorbent at
X* aC*
(15:11) 208C. The experimental procedure involved the following:
¼
X m 1 þ aC*
(1) placing 4000 mL of distilled water in each of 28 flasks,
with each flask having a different initial concentration of dye;
Equation 15.11 is the usual expression for the Langmuir (2) placing the flasks in a water bath held at 208C (using a
isotherm. The equation is well known and can be derived heat-exchanger that recirculates the water in the bath) and
also from a statistical thermodynamics approach. setting up a continuous stirrer for each flask; (3) at time
t ¼ 0, adding 1.00 g of Dowex 50 resin to each flask; (4) meas-
15.2.1.2.1 Plots uring liquid-phase concentrations of Rhodamine-B until no
Figure 15.6a depicts Equation 15.11 graphically, i.e., solid- further change was noted. Dowex 50 resin was used as an
phase equilibrium concentration, X, versus aqueous-phase adsorbent because of the high uptake of Rhodamine-B, and
equilibrium concentration, C*; X m is the maximum solid- that the resin was a light-beige color which stained red in
phase concentration for the particular adsorbate used. The proportion to its uptake to the solid phase. Since the study
plotted points are experimental data obtained from 4 L stirred cited for this illustration was to try to discern a kinetic relation
flasks operated for a time duration such that the aqueous- for adsorption uptake and involved a great many experimental
8 • 10 5 5 • 10 –4
7 • 10 5 1 – =0.0000115+ 0.00000132C*
* *
C X
4 • 10 –4
6 • 10 5
–
5 • 10 5 X*=758018 0.115C* –4
1 +0.115C* 3 • 10
4 • 10 5
3 • 10 5 2 • 10 –4
2 • 10 5
1 • 10 –4
1 • 10 5 1
αX m
0 • 10 0 0 • 10 0
0 50 100 150 200 250 300 350 0 50 100 150 200 250 300 350
(a) C* (μg adsorbate/mL solution) (b) C* (μg adsorbate/mL)
FIGURE 15.6 Langmuir isotherm fit to experimental equilibrium data; T ¼ 208C; adsorbate Rhodamine-B dye; adsorbent Dowex 50 resin
Hþ; X* ¼ 758018[0.115C*=(1 þ 0.115C*)]. (a) Arithmetic Langmuir isotherm. (b) Linearized Langmuir equation. (Adapted from Hendricks,
D.W. and Kuratti, L.C., Kinetics Part I—A laboratory investigation of six proposed rate laws using batch reactors, Utah Water Research
Laboratory, Utah State University, Report PRWG66-1, p. 58, July 59, 1973.)