Page 633 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 633
588 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
. Within a stripping bubble, the partial pressure of gas The idea is to create a large air–water interface for uptake of
‘‘A’’ (the gas being stripped from solution) will dissolved gases from the water by the airflow. The water flow
increase as the bubble rises, that is, it is not zero as must be less than some critical value, above which will cause
assumed in Equation 18.60. flooding. The water flow will pool at the bottom of the reactor
. The stream of air bubbles used for stripping will and flow out under a hydraulic gradient. The airflow enters
increase in concentration of the gas ‘‘A’’ being presumably without any of the gases to be stripped. As the
stripped such that the mass flux of ‘‘A’’ lost from airflow comes in contact with water surfaces, gas transfer
solution equals the mass flux increase to the air occurs from the dissolved gases in aqueous solution to an air
stream. solution. Presumably, the gradients across the films are steep,
. Theoretically, the mass-transfer coefficient, K L a, due to these new air–water interfaces being continuously cre-
should be the same for different gases, differing by ated as the water falls and as the air stream passes across these
their respective diffusion coefficients. surfaces. For stripping a volatile gas, for example, benzene, the
. For moderately soluble gases, K L a once determined higher the airflow the higher the rate of gas transfer. Generally,
for a given gas, for example, oxygen, may be cor- packed towers are used with countercurrent flow, that is, water
rected to use for another gas (used in the same trickles down and air flows up. The air-to-water flow ratios
system and for the same conditions) by the ratio of range 10:1 j(air)=j(water) 300:1.
diffusion coefficients for the two gases. This is based
on substituting in Equation 18.20 the relation, 18.2.3.4.1 Mathematical Modeling
K L ¼ D=d, to show the diffusion coefficient As seen by the depictions in Figure 18.12b, the dissolved
gas concentration decreases with distance along the column.
D A By definition, the reactor is not homogeneous and must be
(18:61)
K L a ¼
d V modeled by selecting an infinitesimal ‘‘slice’’ of the column, as
indicated. Thus, Equation 18.54, the general materials balance
relation is valid, as is Equation 18.56, that is, for the steady state.
18.2.3.4 Column Reactor Modeling: Packed Beds
These equations, per se, are not applied directly, however,
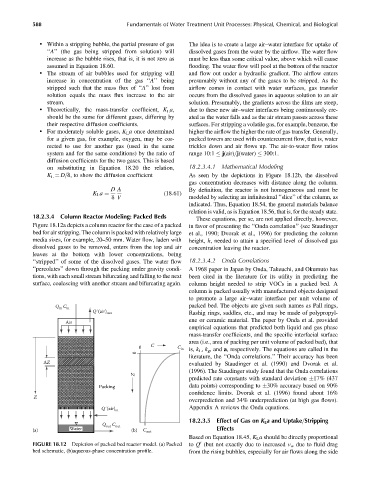
Figure 18.12a depicts a column reactor for the case of a packed in favor of presenting the ‘‘Onda correlation’’ (see Staudinger
bed for air stripping. The column is packed with relatively large et al., 1990; Dvorak et al., 1996) for predicting the column
media sizes, for example, 20–50 mm. Water flow, laden with height, h, needed to attain a specified level of dissolved gas
dissolved gases to be removed, enters from the top and air concentration leaving the reactor.
leaves at the bottom with lower concentrations, being
‘‘stripped’’ of some of the dissolved gases. The water flow 18.2.3.4.2 Onda Correlations
‘‘percolates’’ down through the packing under gravity condi- A 1968 paper in Japan by Onda, Takeuchi, and Okumuto has
tions, with each small stream bifurcating and falling to the next been cited in the literature for its utility in predicting the
surface, coalescing with another stream and bifurcating again. column height needed to strip VOCs in a packed bed. A
column is packed usually with manufactured objects designed
to promote a large air–water interface per unit volume of
packed bed. The objects are given such names as Pall rings,
Q in C in
Rashig rings, saddles, etc., and may be made of polypropyl-
Q΄(air) out
ene or ceramic material. The paper by Onda et al. provided
Air
empirical equations that predicted both liquid and gas phase
mass-transfer coefficients, and the specific interfacial surface
area (i.e., area of packing per unit volume of packed bed), that
0 C C in is, k L , k g ,and a, respectively. The equations are called in the
0
literature, the ‘‘Onda correlations.’’ Their accuracy has been
ΔZ evaluated by Staudinger et al. (1990) and Dvorak et al.
(1996). The Staudinger study found that the Onda correlations
Z
predicted rate constants with standard deviation 17% (437
Packing data points) corresponding to 30% accuracy based on 90%
confidence limits. Dvorak et al. (1996) found about 16%
Z
overprediction and 34% underprediction (at high gas flows).
Q΄(air) in Appendix A reviews the Onda equations.
18.2.3.5 Effect of Gas on K L a and Uptake=Stripping
Q out C out
(a) Water (b) C out Effects
Based on Equation 18.45, K L a should be directly proportional
FIGURE 18.12 Depiction of packed bed reactor model. (a) Packed to Q (but not exactly due to increased v w due to fluid drag
0
bed schematic, (b)aqueous-phase concentration profile. from the rising bubbles, especially for air flows along the side