Page 135 - Gas Purification 5E
P. 135
Alhnolaminesfor Hvdrogen SulJide and Carbon Dioxide Removal 125
10
GAS COMPOSTTION, X ACID GAS
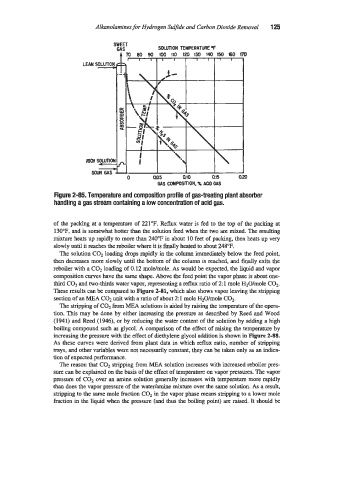
Figure 2-85. Temperature and composition profile of gas-treating plant absorber
handling a gas stream containing a low concentration of acid gas.
of the packing at a temperature of 221°F. Reflux water is fed to the top of the packing at
130"F, and is somewhat hotter than the solution feed when the two are mixed. The resulting
mixtwe heats up rapidly to more than 240°F in about 10 feet of packing, then heats up very
slowly until it reaches the reboiler where it is finally heated to about 248°F.
The solution COz loading drops rapidly in the column immediately below the feed point,
then decreases more slowly until the bottom of the column is reached, and finally exits the
reboiler with a COz loading of 0.12 moldmole. As would be expected, the liquid and vapor
composition curves have the same shape. Above the feed point the vapor phase is about one-
third COl and two-thirds water vapor, representing a reflux ratio of 2 1 mole H2O/mole CO,.
These results can be compared to Figure 2-81, which also shows vapor leaving the stripping
section of an MEA C02 unit with a ratio of about 2: 1 mole H20/mole C02.
The stripping of COz from MEA solutions is aided by raising the temperature of the opera-
tion. This may be done by either increasing the pressure as described by Reed and Wood
(1941) and Reed (1946), or by reducing the water content of the solution by adding a high
boiling compound such as glycol. A comparison of the effect of raising the temperature by
increasing the pressure with the effect of diethylene glycol addition is shown in Figure 2-88.
As these curves were derived fmm plant data in which reflux ratio, number of stripping
trays, and other variables were not necessarily constant, they can be taken only as an indica-
tion of expected performance.
The reason that CO, smpping from MEA solution increases with increased reboiler pres-
sure can be explained on the basis of the effect of temperature on vapor pressures. The vapor
pressure of C02 over an amine solution generally increases with temperature more rapidly
than does the vapor pressure of the water/amine mixture over the same solution. As a result,
stripping to the same mole fraction C02 in the vapor phase means stripping to a lower mole
fraction in the liquid when the pressure (and thus the boiling point) are raised. It should be