Page 130 - Gas Purification 5E
P. 130
120 Gas Purification
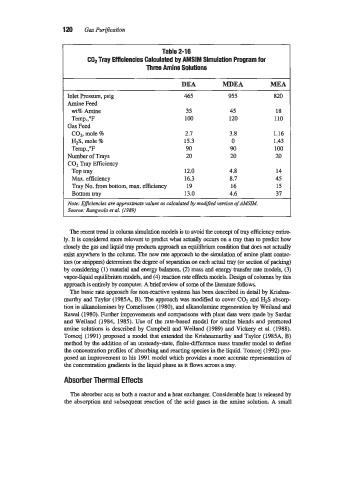
Table 2-1 6
COP Tray Efficiencies Calculated by AMSIM Simulation Program for
Three Amine Solutions
DEA MDEA MEA
Inlet Pressure, psig 465 955 820
Amine Feed
wt% Amine 35 45 18
Temp.,"F 100 120 110
Gas Feed
CO1, mole % 2.7 3.8 1.16
H2S, mole % 15.3 0 1.43
Temp.,"F 90 90 100
Number of Trays 20 20 20
CO1 Tray Efficiency
TOP tray 12.0 4.8 14
Max. efficiency 16.3 8.7 45
Tray No. from bottom, max. efficiency 19 16 15
Bottom tray 13.0 4.6 37
Note: Eficiencies are approximate values as calculated by modified version ofAMSZM.
Source: Rangwala et al. (1989)
The recent trend in column simulation models is to avoid the concept of tray efficiency en&
ly. It is considered more relevant to predict what actually occurs on a tray than to predict how
closely the gas and liquid tray products approach an equilibrium condition that does not actually
exist anywhere in the column. The new rate approach to the simulation of amine plant contac-
tors (or strippers) determines the degree of separation on each actual tray (or section of packing)
by considering (1) material and energy balances, (2) mass and energy transfer rate models, (3)
vapor-liquid equilibrium models, and (4) reaction rate effects models. Design of columns by this
approach is entirely by computer. A brief review of some of the literature follows.
The basic rate approach for non-reactive systems has been described in detail by Krishna-
murthy and Taylor (1985A, B). The approach was modified to cover CO, and H2S absorp-
tion in alkanolamines by Cornelissen (1980), and alkanolamine regeneration by Weiland and
Rawal(l980). Further improvements and comparisons with plant data were made by Sardar
and Weiland (1984, 1985). Use of the rate-based model for amine blends and promoted
amine solutions is described by Campbell and Weiland (1989) and Vickery et al. (1988).
Tomcej (1991) proposed a model that extended the Krishnamurthy and Taylor (1985A, B)
method by the addition of an unsteady-state, finitedifference mass transfer model to define
the concentration profiles of absorbing and reacting species in the liquid. Tomcej (1992) pro-
posed an improvement to his 1991 model which provides a more accurate representation of
the concentration gradients in the liquid phase as it flows across a tray.
Absorber Thermal Effects
The absorber acts as both a reactor and a heat exchanger. Considerable heat is released by
the absorption and subsequent reaction of the acid gases in the amine solution. A small