Page 131 - Gas Purification 5E
P. 131
dlkanolamines for Hydrogen S@de and Carbon Dioxide Removal 121
amount of heat may also be released (or absorbed) by the condensation (or evaporation) of
water vapor. To avoid hydrocarbon condensation the lean solution is usually fed into the top
of the absorber at a slightly higher temperature than that of the sour gas, which is fed into the
bottom. As a result, heat would be transferred from the liquid to the gas even in the absence
of acid gas absorption. The heat of reaction is generated in the liquid phase, which raises the
liquid temperature and causes further heat transfer to the gas. However, the bulk of the
absorption (and therefore heat generation) normally occurs near the bottom of the column, so
the gas is first heated by the liquid near the bottom of the column, then cooled by the incom-
ing lean solution near the top of the column.
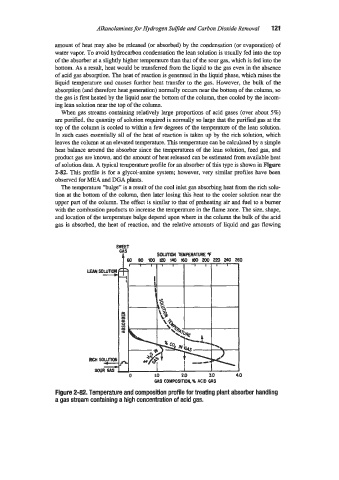
When gas streams containing relatively large proportions of acid gases (over about 5%)
are purified. the quantity of solution required is normally so large that the purified gas at the
top of the column is cooled to within a few degrees of the temperature of the lean solution.
In such cases essentially all of the heat of reaction is taken up by the rich solution, which
leaves the column at an elevated temperature. This temperature can be calculated by a simple
heat balance around the absorber since the temperatures of the lean solution, feed gas, and
product gas are known, and the amount of heat released can be estimated from available heat
of solution data. -4 typical temperature profile for an absorber of this type is shown in Figure
2-82. This profile is for a glycol-amine system; however, very similar profiles have been
observed for ME.4 and DGA plants.
The temperature “bulge” is a result of the cool inlet gas absorbing heat from the rich solu-
tion at the bottom of the column, then later losing this heat to the cooler solution near the
upper part of the column. The effect is similar to that of preheating air and fuel to a burner
with the combustion products to increase the temperature in the flame zone. The size, shape,
and location of the temperature bulge depend upon where in the column the bulk of the acid
gas is absorbed, the heat of reaction, and the relative amounts of liquid and gas flowing
Figure 2-82. Temperature and composition profile for treating plant absoher handling
a gas stream containing a high concentration of acid gas.