Page 133 - Gas Purification 5E
P. 133
Alkanolamines for Hvdrogen Sulfide and Carbon Dioxide Removal 123
1-
2-
3-
4-
5-
6-
7-
F 8-
B
1 ::-
L
P 12-
19-
14 -
15 -
16 -
17 -
80 100 120 140 180 180
Temprature, T
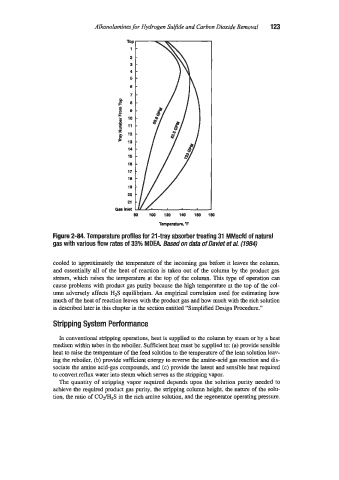
Figure 2-84. Temperature profiles for 21 -tray absorber treating 31 MMscfd of natural
gas with various flow rates of 33% MDEA. Based on data of Daviet ef a/. (1984)
cooled to approximately the temperature of the incoming gas before it leaves the column,
and essentially all of the heat of reaction is taken out of the column by the product gas
stream, which raises the temperature at the top of the column. This type of operation can
cause problems with product gas purity because the high temperature at the top of the col-
umn adversely affects HIS equilibrium. An empirical correlation used for estimating h0~7
much of the heat of reaction leaves with the product gas and how much with the rich solution
is described later in this chapter in the section entitled ‘Simplified Design Procedure.’‘
Stripping System Performance
In conventional stripping operations, heat is supplied to the column by steam or by a heat
medium within tubes in the reboiler. Sufficient heat must be supplied to: (a) provide sensible
heat to raise the temperature of the feed solution to the temperature of the lean solution leav-
ing the reboiler, (b) provide sufficient energy to reverse the amine-acid gas reaction and dis-
sociate the amine acid-gas compounds, and (c) provide the latent and sensible heat required
to convert reflux water into steam which serves as the stripping vapor.
The quantity of stripping vapor required depends upon the solution purity needed to
achieve the required product gas purity, the stripping column height, the nature of the solu-
tion, the ratio of C02/H2S in the rich amine solution, and the regenerator operating pressure.