Page 128 - Gas Purification 5E
P. 128
1 18 Gas Plir$cation
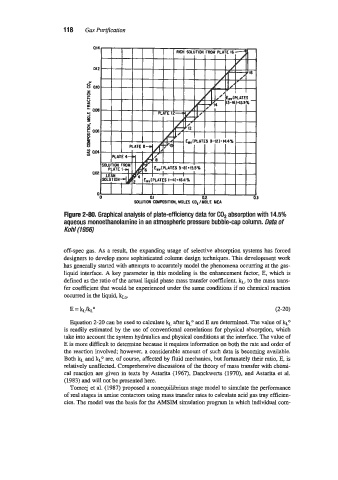
Figure 2-80. Graphical analysis of plate-efficiency data for COP absorption with 14.5%
aqueous monoethanolamine in an atmospheric pressure bubble-cap column. Data of
Kohl (1956)
off-spec gas. As a result, the expanding usage of selective absorption systems has forced
designers to develop more sophisticated column design techniques. This development work
has generally started with attempts to accurately model the phenomena occurring at the gas-
liquid intefiace. A key parameter in this modeling is the enhancement factor, E, which is
defined as the ratio of the actual liquid phase mass transfer coefficient, kL, to the mass trans-
fer coefficient that would be experienced under the same conditions if no chemical reaction
occurred in the liquid, kLo.
E = kL/kLo (2-20)
Equation 2-20 can be used to calculate kL after ho and E are determined. The value of ko
is readily estimated by the use of conventional correlations for physical absorption, which
take into account the system hydraulics and physical conditions at the interface. The value of
E is more difficult to determine because it requires information on both the rate and order of
the reaction involved; however! a considerable amount of such data is becoming available.
Both kL and ko are, of course, affected by fluid mechanics, but fortunately their ratio, E, is
relatively unaffected. Comprehensive discussions of the theory of mass transfer with chemi-
cal reaction are given in texts by Astarita (1967), Danckwerts (1970), and Astarita et al.
(1983) and will not be presented here.
Tomcej et al. (1987) proposed a nonequilibrium stage model to simulate the performance
of real stages in amine contacfors using mass transfer rates to calculate acid gas tray efficien-
cies. The model was the basis for the AMSIM simulation program in which individual com-