Page 248 - Gas Purification 5E
P. 248
234 Gas PuriJcation
to Meisen and Kennard (1982), the extent of these reactions can be limited by avoiding ele-
vated temperatures. Reboiler heat flux should be limited, and amine circulation through the
reboiler should be kept high. Amine regenerator operating temperatures may be limited by
the need to minimize amine degradation (Polderman and Steele, 1956). Kim and Sartori
(1984) have also shown that the degradation reactions of DEA depend on the C02 solution
loading (i.e., the equilibrium COz partial pressure above the rich solution, and the amine
concentration), but are not affected by the presence of H2S. This suggests that these reactions
may impose additional limitations on the rich amine solution CO, loading and the amine
solution concentration for MEA, DGA, DIPA, and DEA. MDEA is not affected by high C02
loadings because there are no C02-MDEA degradation reactions.
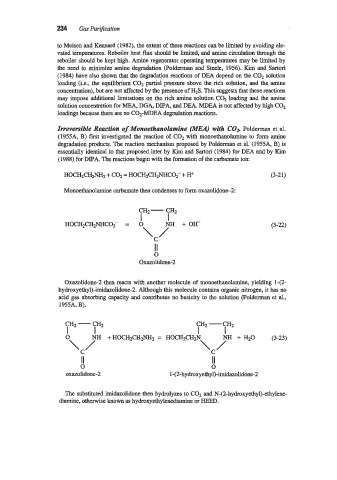
Irreversible Reaction of Monoethanolamine (MEA) with COP Polderman et al.
(1955A, B) first investigated the reaction of COz with monoethanolamine to form amine
degradation products. The reaction mechanism proposed by Polderman et al. (1955A, B) is
essentially identical to that proposed later by Kim and Sartori (1984) for DEA and by Kim
(1988) for DIPA. The reactions begin with the formation of the carbamate ion:
HOCHzCHzNHz + C02 = HOCH2CHzNHCOz- + H+ (3-21)
Monoethanolamine carbamate then condenses to form oxazolidone-2:
CH2 -
CH2
I I
HOCHzCHzNHCO2- = 0 (3-22)
C
\ 7 +OH
II
0
Oxazolidone-2
Oxazolidone-2 then reacts with another molecule of monoethanolamine, yielding 1-(2-
hydroxyethyl)-imidazolidone-2. Although this molecule contains organic nitrogen, it has no
acid gas absorbing capacity and contributes no basicity to the solution (Polderman et al.,
1955A, B).
CH2 - CH2 -
CH2
CH2
I I I I
0 NH + HOCHzCHzNHz = HOCH2CH2N (3-23)
\/ \ i” + H20
C
C
II II
0 0
oxazolidone-2 1 -( 2-hydroxyethyl)-imidazolidone-2
The substituted imidazolidone then hydrolyzes to C02 and N-(2-hydroxyethyl)-ethylene-
diamine, otherwise known as hydroxyethylenediamine or HEED.