Page 250 - Gas Purification 5E
P. 250
236 Gas Punpcation
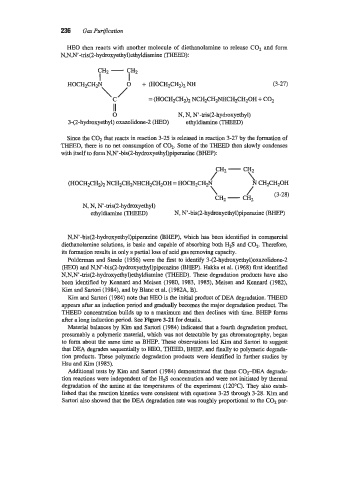
HE0 then reacts with another molecule of diethanolamine to release C02 and form
N,N,N’-tris(2-hydroxyethyl)ethyldiamine (THEED):
‘
CH2 - CH2
I I
NH
HOCH2CH2N /” + (HOCH~CHZ)~ (3-27)
C
= (HOCH2CH& NCH2CH2NHCH2CH20H + COz
II
0 N, N, N’-tris(2-hydroxyethyl)
3-(2-hydroxyethyl) oxazolidone-2 (HEO) ethyldiamine (THEED)
Since the C02 that reacts in reaction 3-25 is released in reaction 3-27 by the formation of
THEED, there is no net consumption of COP Some of the =ED then slowly condenses
with itself to form N,N’-bis(2-hydroxyethyl)piperazine (BHEP):
(HOCH2CH2)2 NCH~CH~NHCHZCH~OH HOCHzCH2N 7-7 ,N CH2CW:”,
=
\ CH2
CH2 -
N, N, N’-tris(2-hydroxyethyl)
ethyldiamine (THEED) N, N’-bis(2-hydroxyethyl)piperazine (BHEP)
N,N’-bis(2-hydroxyethyl)piperazine (BHEP), which has been identified in commercial
diethanolamine solutions, is basic and capable of absorbing both H2S and COP Therefore,
its formation results in only a partial loss of acid gas removing capacity.
Polderman and Steele (1956) were the first to identify 3-(2-hydroxyethyl)oxazolidone-2
(HEO) and N,N’-bis(2-hydroxyethyl)piperazine (BHEP). Hakka et al. (1968) first identified
N,N,N’-tris(2-hydroxyethyl)ethyldiamine (THEED). These degradation products have also
been identified by Kennard and Meisen (1980, 1983, 1985), Meisen and Kennard (1982),
Kim and Sartori (1984), and by Blanc et al. (1982A, €3).
Kim and Sartori (1984) note that HE0 is the initial product of DEA degradation. THEED
appears after an induction period and gradually becomes the major degradation product. The
THEED concentration builds up to a maximum and then declines with time. BHEP forms
after a long induction period. See Figure 3-21 for details.
Material balances by Kim and Sartori (1984) indicated that a fourth degradation product,
presumably a polymeric material, which was not detectable by gas chromatography, began
to form about the same time as BHEP. These observations led Kim and Sartori to suggest
that DEA degrades sequentially to HEO, THEED, BHEP, and finally to polymeric degrada-
tion products. These polymeric degradation products were identified in further studies by
Hsu and Kim (1985).
Additional tests by Kim and Sartori (1984) demonstrated that these C02-DEA degrada-
tion reactions were independent of the H2S concentration and were not initiated by thermal
degradation of the amine at the temperatures of the experiment (120°C). They also estab-
lished that the reaction kinetics were consistent with equations 3-25 through 3-28. Kim and
Sartori also showed that the DEA degradation rate was roughly proportional to the C02 par-