Page 251 - Gas Purification 5E
P. 251
Mechanical Design and Operation of Alkanolamine Plants 237
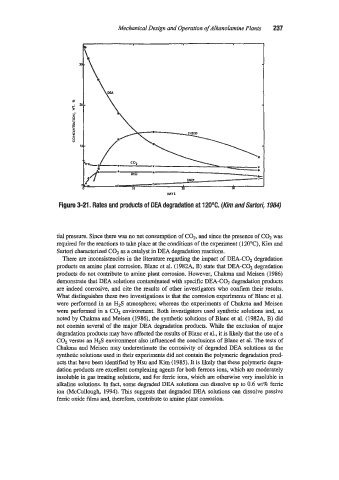
Figure 3-21. Rates and products of DEA degradation at 120°C. (Kim and Sartofi, 1984)
tial pressure. Since there was no net consumption of COz, and since the presence of C02 was
required for the reactions to take place at the conditions of the experiment ( 120°C), Kim and
Sartori characterized COz as a catalyst in DEA degradation reactions.
There are inconsistencies in the literature regarding the impact of DEA-C02 degradation
products on amine plant corrosion. Blanc et al. (1982A, B) state that DEA-C02 degradation
products do not contribute to amine plant corrosion. However, Chakma and Meisen (1986)
demonstrate that DEA solutions contaminated with specific DEA-C02 degradation products
are indeed corrosive, and cite the results of other investigators who con- their results.
What distinguishes these two investigations is that the corrosion experiments of Blanc et al.
were performed in an HzS atmosphere; whereas the experiments of Chakma and Meisen
were performed in a COz environment. Both investigators used synthetic solutions and, as
noted by Chakma and Meisen (1986), the synthetic solutions of Blanc et al. (1982A, B) did
not contain several of the major DEA degradation products. While the exclusion of major
degradation products may have affected the results of Blanc et al., it is likely that the use of a
C02 versus an H2S environment also influenced the conclusions of Blanc et al. The tests of
Chakma and Meisen may underestimate the corrosivity of degraded DEA solutions as the
synthetic solutions used in their experiments did not contain the polymeric degradation prod-
ucts that have been identified by Hsu and Kim (1985). It is likely that these polymeric degra-
dation products are excellent complexing agents for both ferrous ions, which are moderately
insoluble in gas treating solutions, and for ferric ions, which are otherwise very insoluble in
alkaline solutions. In fact, some degraded DEA solutions can dissolve up to 0.6 wt% ferric
ion (McCullough, 1994). This suggests that degraded DEA solutions can dissolve passive
ferric oxide films and, therefore, contribute to amine plant corrosion.