Page 249 - Gas Purification 5E
P. 249
Mechanical Design and Operation of Alkanolamine Plants 235
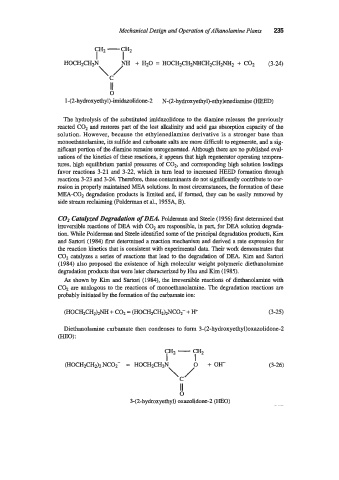
CH2 -
CH2
I I
HOCHzCHzN NH + H20 = HOCH2CH2NHCH2CH2NH2 + COz (3-24)
\/
C
II
0
1-(2-hydroxyethyl)-imidazolidone-2 N-(2-hydroxyethyl)-ethylenediamine (HEED)
The hydrolysis of the substituted imidazolidone to the diamine releases the previously
reacted COz and restores part of the lost alkalinity and acid gas absorption capacity of the
solution. However, because the ethylenediamine derivative is a stronger base than
monoethanolamine, its sulfide and carbonate salts are more difficult to regenerate, and a sig-
nificant portion of the diamine remains unregenerated. Although there are no published eval-
uations of the kinetics of these reactions, it appears that high regenerator operating tempera-
tures, high equilibrium partial pressures of COz, and corresponding high solution loadings
favor reactions 3-21 and 3-22, which in turn lead to increased HEED formation through
reactions 3-23 and 3-24. Therefore, these contaminants do not significantly contribute to cor-
rosion in properly maintained MEA solutions. In most circumstances, the formation of these
MEA-C02 degradation products is limited and, if formed, they can be easily removed by
side stream reclaiming (Polderman et al., 1955A, B).
C02 Catalyzed Degradation of DEA. Polderman and Steele (1956) first determined that
irreversible reactions of DEA with COz are responsible, in part, for DEA solution degrada-
tion. While Polderman and Steele identified some of the principal degradation products, Kim
and Sartori (1984) first determined a reaction mechanism and derived a rate expression for
the reaction kinetics that is consistent with experimental data. Their work demonstrates that
COz catalyzes a series of reactions that lead to the degradation of DEA. Kim and Sartori
(1984) also proposed the existence of high molecular weight polymeric diethanolamine
degradation products that were later characterized by Hsu and Kim (1985).
As shown by Kim and Sartori (1984), the irreversible reactions of diethanolamine with
C02 are analogous to the reactions of monoethanolamine. The degradation reactions are
probably initiated by the formation of the carbamate ion:
(HOCH2CHz)zNH + COZ = (HOCH2CH2)2NCOZ- + H+ (3-25)
Diethanolamine carbamate then condenses to form 3-(2-hydroxyethyl)oxazolidone-2
(HEO):
CH2 - CH2
I I
(HOCH2CH2)2 NC02- = HOCH2CH2N (3-26)
C
\ /" +OH
It
0
3-(2-hydroxyethyl) oxazolidone-2 (HEO)