Page 64 - Gas Purification 5E
P. 64
54 Gas PuriJcation
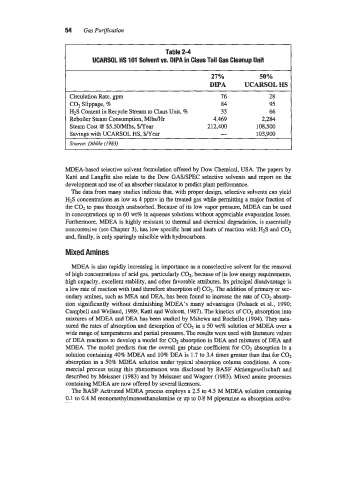
I UCARSOL HS 101 Solvent vs. DIPA in Claus Tail Gas Cleanup Unit
Table 2-4
27%
DIPA UCARSOL HS
Circulation Rate, gpm 76 28
CO-, Slippage, 8 84 95
H2S Content in Recycle Stream to Claus Unit, 76 35 66
Reboiler Steam Consumption, Mlbs/Hr 4,469 2,284
Steam Cost @ $5.50/Mlbs, $/Year 212,300 108,500
Savings with UCARSOL HS, $/Year - 103,900
Source: Dibble (1983)
h4DEA-based selective solvent formulation offered by Dow Chemical, USA. The papers by
Katti and Langfitt also relate to the Dow GAS/SPEC selective solvents and report on the
development and use of an absorber simulator to predict plant performance.
The data from many studies indicate that, with proper design, selective solvents can yield
H2S concentrations as low as 4 ppmv in the treated gas while permitting a major fraction of
the C02 to pass through unabsorbed. Because of its low vapor pressure, MDEA can be used
in concentrations up to 60 wt% in aqueous solutions without appreciable evaporation losses.
Furthermore. MDEA is highly resistant to thermal and chemical de,pdation. is essentially
noncorrosive (see Chapter 3): has low specific heat and heats of reaction with H2S and CO-,
and, finally, is only sparingly miscible with hydrocarbons.
Mixed Amines
MDEA is also rapidly increasing in importance as a nonselective solvent for the removal
of high concentrations of acid gas, particularly CO2, because of its low energy requirements,
high capacity, excellent stability, and other favorable attributes. Its principal disadvantage is
a low rate of reaction with (and therefore absorption of) CO-,. The addition of primary or sec-
ondary amines, such as MEA and DEA, has been found to increase the rate of COS absorp-
tion significantly without diminishing MDEA’s many advantages (Polasek et al., 1990;
Campbell and Weiland, 1989: Katti and Wolcott, 1987). The kinetics of COz absorption into
mixtures of MDEA and DEA has been studied by Mshewa and Rochelle (1994). They mea-
sured the rates of absorption and desorption of C02 in a 50 wt%, solution of MDEA over a
wide range of temperatures and partial pressures. The results were used with literature values
of DEA reactions to develop a model for C02 absorption in DEA and mixtures of DEA and
MDEA. The model predicts that the overall gas phase coefficient for CO-, absorption in a
solution containing 40% MDEA and 10% DEA is 1.7 to 3.4 times greater than that for C02
absorption in a 50% MDEA solution under typical absorption column conditions. A com-
mercial process using this phenomenon was disclosed by BASF Aktiengesellschaft and
described by Meissner (1983) and by Meissner and Wagner (1983). Mixed amine processes
containing MDEA are now offered by several licensors.
The BASF Activated MDEA process employs a 2.5 to 4.5 M MDEA solution containing
0.1 to 0.4 M monomethylmonoethanolamine or up to 0.8 M piperazine as absorption activa-