Page 255 - gas transport in porous media
P. 255
Šolcová and Schneider
252
cell. Different gases A and B flow steadily through the both compartments until
the steady state is established. Inlet flow-rates of gases A and B should be high
enough to guarantee that gases flowing from both compartments are nearly pure (i.e.,
L
L
y U = y = 1 and y = y U = 0). After the gas inlet and outlet in one of the cell
A B A B
compartment is stopped, the net volumetric diffusion flux is determined. For this
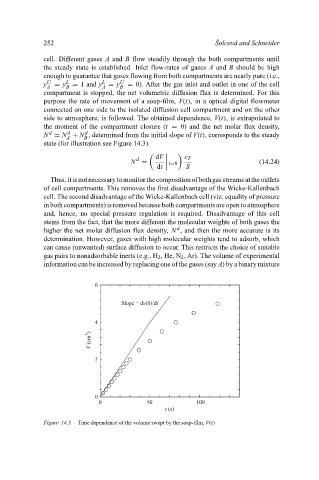
purpose the rate of movement of a soap-film, V(t), in a optical digital flowmeter
connected on one side to the isolated diffusion cell compartment and on the other
side to atmosphere, is followed. The obtained dependence, V(t), is extrapolated to
the moment of the compartment closure (t = 0) and the net molar flux density,
d
d
d
N = N + N , determined from the initial slope of V(t), corresponds to the steady
A B
state (for illustration see Figure 14.3).
d dV c T
N = t=0 (14.24)
dt S
Thus, itisnotnecessarytomonitorthecompositionofbothgasstreamsattheoutlets
of cell compartments. This removes the first disadvantage of the Wicke-Kallenbach
cell. The second disadvantage of the Wicke-Kallenbach cell (viz. equality of pressure
in both compartments) is removed because both compartments are open to atmosphere
and, hence, no special pressure regulation is required. Disadvantage of this cell
stems from the fact, that the more different the molecular weights of both gases the
d
higher the net molar diffusion flux density, N , and then the more accurate is its
determination. However, gases with high molecular weights tend to adsorb, which
can cause (unwanted) surface diffusion to occur. This restricts the choice of suitable
gas pairs to nonadsorbable inerts (e.g., H 2 , He, N 2 , Ar). The volume of experimental
information can be increased by replacing one of the gases (say A) by a binary mixture
6
Slope = dv(0)/dt
4
V (cm 3 )
2
0
0 50 100
t (s)
Figure 14.3. Time dependence of the volume swept by the soap-film, V(t)