Page 357 - gas transport in porous media
P. 357
Chapter 22: Environmental Remediation of Volatile Organic Compounds
r 1 359
l v
r 2
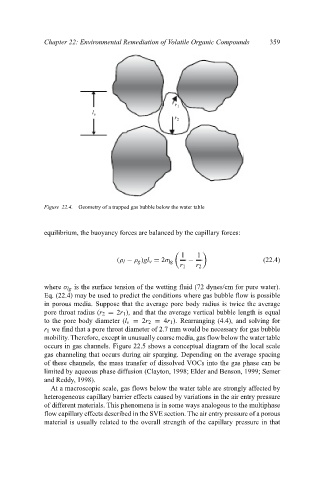
Figure 22.4. Geometry of a trapped gas bubble below the water table
equilibrium, the buoyancy forces are balanced by the capillary forces:
1 1
(ρ l − ρ g )gl v = 2σ lg − (22.4)
r 1 r 2
where σ lg is the surface tension of the wetting fluid (72 dynes/cm for pure water).
Eq. (22.4) may be used to predict the conditions where gas bubble flow is possible
in porous media. Suppose that the average pore body radius is twice the average
pore throat radius (r 2 = 2r 1 ), and that the average vertical bubble length is equal
to the pore body diameter (l v = 2r 2 = 4r 1 ). Rearranging (4.4), and solving for
r 1 we find that a pore throat diameter of 2.7 mm would be necessary for gas bubble
mobility. Therefore, except in unusually coarse media, gas flow below the water table
occurs in gas channels. Figure 22.5 shows a conceptual diagram of the local scale
gas channeling that occurs during air sparging. Depending on the average spacing
of these channels, the mass transfer of dissolved VOCs into the gas phase can be
limited by aqueous phase diffusion (Clayton, 1998; Elder and Benson, 1999; Semer
and Reddy, 1998).
At a macroscopic scale, gas flows below the water table are strongly affected by
heterogeneous capillary barrier effects caused by variations in the air entry pressure
of different materials. This phenomena is in some ways analogous to the multiphase
flow capillary effects described in the SVE section. The air entry pressure of a porous
material is usually related to the overall strength of the capillary pressure in that