Page 361 - gas transport in porous media
P. 361
Chapter 22: Environmental Remediation of Volatile Organic Compounds
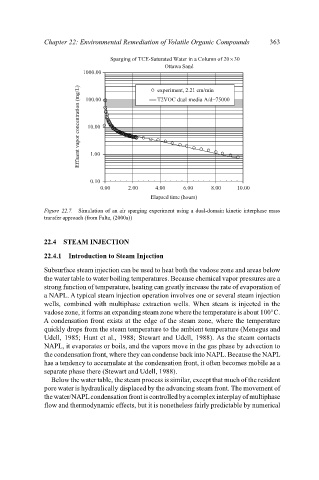
Sparging of TCE-Saturated Water in a Column of 20×30
Ottawa Sand 363
1000.00 experiment, 2.21 cm/min
Effluent vapor concentration (mg/L) 10.00
T2VOC dual media A/d=75000
100.00
1.00
0.10
0.00 2.00 4.00 6.00 8.00 10.00
Elapsed time (hours)
Figure 22.7. Simulation of an air sparging experiment using a dual-domain kinetic interphase mass
transfer approach (from Falta, (2000a))
22.4 STEAM INJECTION
22.4.1 Introduction to Steam Injection
Subsurface steam injection can be used to heat both the vadose zone and areas below
the water table to water boiling temperatures. Because chemical vapor pressures are a
strong function of temperature, heating can greatly increase the rate of evaporation of
a NAPL. A typical steam injection operation involves one or several steam injection
wells, combined with multiphase extraction wells. When steam is injected in the
vadose zone, it forms an expanding steam zone where the temperature is about 100 C.
◦
A condensation front exists at the edge of the steam zone, where the temperature
quickly drops from the steam temperature to the ambient temperature (Menegus and
Udell, 1985; Hunt et al., 1988; Stewart and Udell, 1988). As the steam contacts
NAPL, it evaporates or boils, and the vapors move in the gas phase by advection to
the condensation front, where they can condense back into NAPL. Because the NAPL
has a tendency to accumulate at the condensation front, it often becomes mobile as a
separate phase there (Stewart and Udell, 1988).
Below the water table, the steam process is similar, except that much of the resident
pore water is hydraulically displaced by the advancing steam front. The movement of
the water/NAPLcondensation front is controlled by a complex interplay of multiphase
flow and thermodynamic effects, but it is nonetheless fairly predictable by numerical