Page 113 - Geothermal Energy Renewable Energy and The Environment
P. 113
Exploring for Geothermal Systems 99
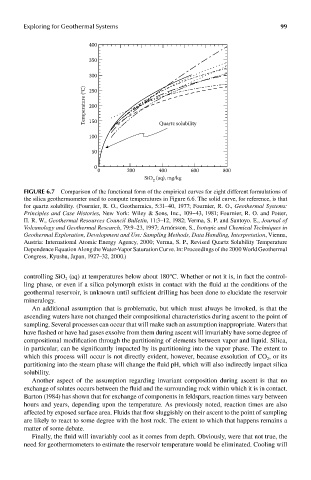
400
350
300
Temperature (°C) 200
250
150
100 Quartz solubility
50
0
0 200 400 600 800
SiO (aq), mg/kg
2
FIGUre 6.7 Comparison of the functional form of the empirical curves for eight different formulations of
the silica geothermometer used to compute temperatures in Figure 6.6. The solid curve, for reference, is that
for quartz solubility. (Fournier, R. O., Geothermics, 5:31–40, 1977; Fournier, R. O., Geothermal Systems:
Principles and Case Histories, New York: Wiley & Sons, Inc., 109–43, 1981; Fournier, R. O. and Potter,
II. R. W., Geothermal Resources Council Bulletin, 11:3–12, 1982; Verma, S. P. and Santoyo. E., Journal of
Volcanology and Geothermal Research, 79:9–23, 1997; Arnórsson, S., Isotopic and Chemical Techniques in
Geothermal Exploration, Development and Use: Sampling Methods, Data Handling, Interpretation, Vienna,
Austria: International Atomic Energy Agency, 2000; Verma, S. P., Revised Quartz Solubility Temperature
Dependence Equation Along the Water-Vapor Saturation Curve. In: Proceedings of the 2000 World Geothermal
Congress, Kyushu, Japan, 1927–32, 2000.)
controlling SiO (aq) at temperatures below about 180°C. Whether or not it is, in fact the control-
2
ling phase, or even if a silica polymorph exists in contact with the fluid at the conditions of the
geothermal reservoir, is unknown until sufficient drilling has been done to elucidate the reservoir
mineralogy.
An additional assumption that is problematic, but which must always be invoked, is that the
ascending waters have not changed their compositional characteristics during ascent to the point of
sampling. Several processes can occur that will make such an assumption inappropriate. Waters that
have flashed or have had gases exsolve from them during ascent will invariably have some degree of
compositional modification through the partitioning of elements between vapor and liquid. Silica,
in particular, can be significantly impacted by its partitioning into the vapor phase. The extent to
which this process will occur is not directly evident, however, because exsolution of CO , or its
2
partitioning into the steam phase will change the fluid pH, which will also indirectly impact silica
solubility.
Another aspect of the assumption regarding invariant composition during ascent is that no
exchange of solutes occurs between the fluid and the surrounding rock within which it is in contact.
Barton (1984) has shown that for exchange of components in feldspars, reaction times vary between
hours and years, depending upon the temperature. As previously noted, reaction times are also
affected by exposed surface area. Fluids that flow sluggishly on their ascent to the point of sampling
are likely to react to some degree with the host rock. The extent to which that happens remains a
matter of some debate.
Finally, the fluid will invariably cool as it comes from depth. Obviously, were that not true, the
need for geothermometers to estimate the reservoir temperature would be eliminated. Cooling will