Page 114 - Geothermal Energy Renewable Energy and The Environment
P. 114
100 Geothermal Energy: Renewable Energy and the Environment
350
300
Computed temperature (°C) 200 Experiment
250
150
100
50
0
0 50 100 150 200 250 300 350
Measured temperature (°C)
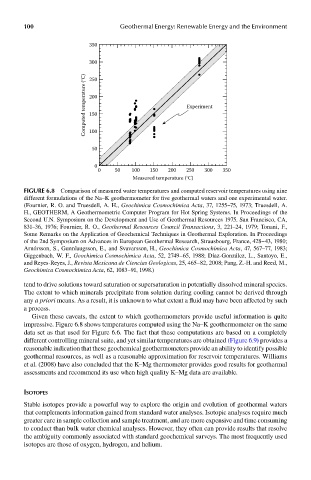
FIGUre 6.8 Comparison of measured water temperatures and computed reservoir temperatures using nine
different formulations of the Na–K geothermometer for five geothermal waters and one experimental water.
(Fournier, R. O. and Truesdell, A. H., Geochimica Cosmochimica Acta, 37, 1255–75, 1973; Truesdell, A.
H., GEOTHERM, A Geothermometric Computer Program for Hot Spring Systems. In Proceedings of the
Second U.N. Symposium on the Development and Use of Geothermal Resources 1975. San Francisco, CA,
831–36, 1976; Fournier, R. O., Geothermal Resources Council Transactions, 3, 221–24, 1979; Tonani, F.,
Some Remarks on the Application of Geochemical Techniques in Geothermal Exploration. In Proceedings
of the 2nd Symposium on Advances in European Geothermal Research, Strausbourg, France, 428–43, 1980;
Arnórsson, S., Gunnlaugsson, E., and Svavarsson, H., Geochimica Cosmochimica Acta, 47, 567–77, 1983;
Giggenbach, W. F., Geochimica Cosmochimica Acta, 52, 2749–65, 1988; Díaz-González, L., Santoyo, E.,
and Reyes-Reyes, J., Revista Mexicana de Ciencias Geologicas, 25, 465–82, 2008; Pang, Z.-H. and Reed, M.,
Geochimica Cosmochimica Acta, 62, 1083–91, 1998.)
tend to drive solutions toward saturation or supersaturation in potentially dissolved mineral species.
The extent to which minerals precipitate from solution during cooling cannot be derived through
any a priori means. As a result, it is unknown to what extent a fluid may have been affected by such
a process.
Given these caveats, the extent to which geothermometers provide useful information is quite
impressive. Figure 6.8 shows temperatures computed using the Na–K geothermometer on the same
data set as that used for Figure 6.6. The fact that these computations are based on a completely
different controlling mineral suite, and yet similar temperatures are obtained (Figure 6.9) provides a
reasonable indication that these geochemical geothermometers provide an ability to identify possible
geothermal resources, as well as a reasonable approximation for reservoir temperatures. Williams
et al. (2008) have also concluded that the K–Mg thermometer provides good results for geothermal
assessments and recommend its use when high quality K–Mg data are available.
isoTopes
Stable isotopes provide a powerful way to explore the origin and evolution of geothermal waters
that complements information gained from standard water analyses. Isotopic analyses require much
greater care in sample collection and sample treatment, and are more expensive and time consuming
to conduct than bulk water chemical analyses. However, they often can provide results that resolve
the ambiguity commonly associated with standard geochemical surveys. The most frequently used
isotopes are those of oxygen, hydrogen, and helium.