Page 116 - Geothermal Energy Renewable Energy and The Environment
P. 116
102 Geothermal Energy: Renewable Energy and the Environment
The values for these ratios can be measured by mass spectrometry, but the absolute values for
each isotope are not routinely measured. Generally, a standard with an assigned or measured isoto-
pic ratio is used as the basis for evaluating other samples of interest. For oxygen and hydrogen iso-
tope measurements the standard has generally been Standard Mean Ocean Water (SMOW), and the
isotopic composition of a sample is expressed as the difference from SMOW in parts per thousand,
16
18
18
18
16
δ O = {[( O/ O) sample − ( O/ O) SMOW ]/( O/ O) SMOW } × 1000
16
18
δD = {[(D/H) sample − (D/H) SMOW ]/(D/H) SMOW } × 1000.
Substances that are isotopically lighter than SMOW will consequently have a negative δ O and
18
δD. Other standards have also been used, resulting in different δ O and δD values. It is thus impor-
18
tant to know what standard was used in an analysis in order to allow quantitative comparison of
different samples.
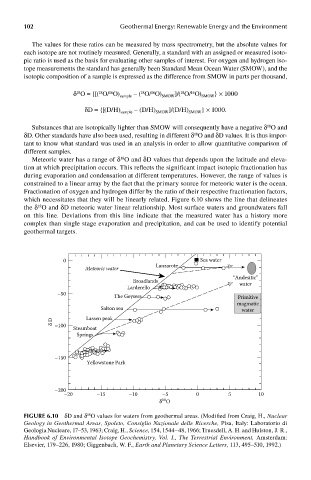
Meteoric water has a range of δ O and δD values that depends upon the latitude and eleva-
18
tion at which precipitation occurs. This reflects the significant impact isotopic fractionation has
during evaporation and condensation at different temperatures. However, the range of values is
constrained to a linear array by the fact that the primary source for meteoric water is the ocean.
Fractionation of oxygen and hydrogen differ by the ratio of their respective fractionation factors,
which necessitates that they will be linearly related. Figure 6.10 shows the line that delineates
the δ O and δD meteoric water linear relationship. Most surface waters and groundwaters fall
18
on this line. Deviations from this line indicate that the measured water has a history more
complex than single stage evaporation and precipitation, and can be used to identify potential
geothermal targets.
0 Sea water
Meteoric water Lanzarote
“Andesitic”
Broadlands
Larderello water
–50 The Geysers Primitive
magmatic
Salton sea water
Lassen peak
δ D –100 Steamboat
Springs
–150
Yellowstone Park
–200
–20 –15 –10 –5 0 5 10
18
δ O
18
FIGUre 6.10 δD and δ O values for waters from geothermal areas. (Modified from Craig, H., Nuclear
Geology in Geothermal Areas, Spoleto, Consiglio Nazionale delle Ricerche, Pisa, Italy: Laboratorio di
Geologia Nucleare, 17–53, 1963; Craig, H., Science, 154, 1544–48, 1966; Truesdell, A. H. and Hulston, J. R.,
Handbook of Environmental Isotope Geochemistry, Vol. I., The Terrestrial Environment, Amsterdam:
Elsevier, 179–226, 1980; Giggenbach, W. F., Earth and Planetary Science Letters, 113, 495–510, 1992.)