Page 203 - Geothermal Energy Renewable Energy and The Environment
P. 203
190 Geothermal Energy: Renewable Energy and the Environment
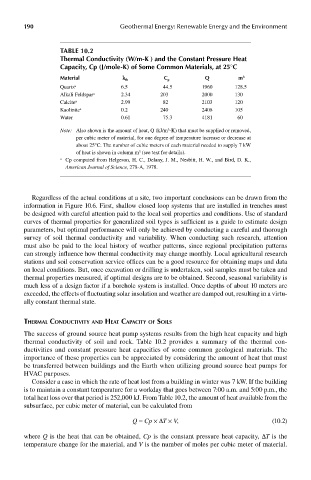
Table 10.2
Thermal conductivity (w/m-k ) and the constant pressure heat
capacity, cp (J/mole-k) of some common materials, at 25°c
material k th c p q m 3
Quartz a 6.5 44.5 1960 128.5
Alkali Feldspar a 2.34 203 2000 130
Calcite a 2.99 82 2103 120
Kaolinite a 0.2 240 2408 105
Water 0.61 75.3 4181 60
3
Note: Also shown is the amount of heat, Q (kJ/m -K) that must be supplied or removed,
per cubic meter of material, for one degree of temperature increase or decrease at
about 25°C. The number of cubic meters of each material needed to supply 7 kW
3
of heat is shown in column m (see text for details).
a Cp computed from Helgeson, H. C., Delany, J. M., Nesbitt, H. W., and Bird, D. K.,
American Journal of Science, 278-A, 1978.
Regardless of the actual conditions at a site, two important conclusions can be drawn from the
information in Figure 10.6. First, shallow closed loop systems that are installed in trenches must
be designed with careful attention paid to the local soil properties and conditions. Use of standard
curves of thermal properties for generalized soil types is sufficient as a guide to estimate design
parameters, but optimal performance will only be achieved by conducting a careful and thorough
survey of soil thermal conductivity and variability. When conducting such research, attention
must also be paid to the local history of weather patterns, since regional precipitation patterns
can strongly influence how thermal conductivity may change monthly. Local agricultural research
stations and soil conservation service offices can be a good resource for obtaining maps and data
on local conditions. But, once excavation or drilling is undertaken, soil samples must be taken and
thermal properties measured, if optimal designs are to be obtained. Second, seasonal variability is
much less of a design factor if a borehole system is installed. Once depths of about 10 meters are
exceeded, the effects of fluctuating solar insolation and weather are damped out, resulting in a virtu-
ally constant thermal state.
Thermal conducTiviTy and heaT capaciTy of soils
The success of ground source heat pump systems results from the high heat capacity and high
thermal conductivity of soil and rock. Table 10.2 provides a summary of the thermal con-
ductivities and constant pressure heat capacities of some common geological materials. The
importance of these properties can be appreciated by considering the amount of heat that must
be transferred between buildings and the Earth when utilizing ground source heat pumps for
HVAC purposes.
Consider a case in which the rate of heat lost from a building in winter was 7 kW. If the building
is to maintain a constant temperature for a workday that goes between 7:00 a.m. and 5:00 p.m., the
total heat loss over that period is 252,000 kJ. From Table 10.2, the amount of heat available from the
subsurface, per cubic meter of material, can be calculated from
Q = Cp × ∆T × V, (10.2)
where Q is the heat that can be obtained, Cp is the constant pressure heat capacity, ∆T is the
temperature change for the material, and V is the number of moles per cubic meter of material.