Page 60 - Geothermal Energy Renewable Energy and The Environment
P. 60
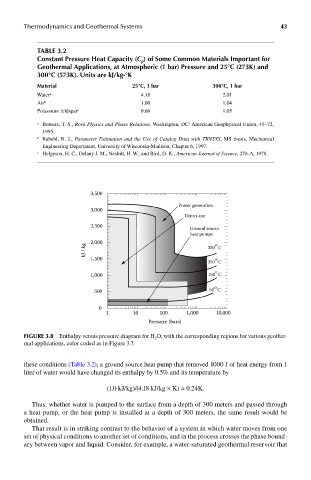
Thermodynamics and Geothermal Systems 43
Table 3.2
constant pressure heat capacity (C ) of some common materials Important for
p
Geothermal applications, at atmospheric (1 bar) pressure and 25°c (273k) and
300°c (573k). Units are kJ/kg-°k
material 25°c, 1 bar 300°c, 1 bar
Water a 4.18 2.01
Air b 1.00 1.04
Potassium feldspar c 0.66 1.05
a Bowers, T. S., Rock Physics and Phase Relations, Washington, DC: American Geophysical Union, 45–72,
1995.
b Rabehl, R. J., Parameter Estimation and the Use of Catalog Data with TRNSYS, MS thesis, Mechanical
Engineering Department, University of Wisconsin-Madison, Chapter 6, 1997.
c Helgeson, H. C., Delany J. M., Nesbitt, H. W., and Bird, D. K., American Journal of Science, 278-A, 1978.
3,500
Power generation
3,000
Direct-use
2,500
Ground source
heat pumps
2,000 o
kJ / kg 350 C
1,500 o
250 C
o
1,000 150 C
o
500 50 C
bars
0
1 10 100 1,000 10,000
Pressure (bars)
FIGUre 3.8 Enthalpy versus pressure diagram for H 2 O, with the corresponding regions for various geother-
mal applications, color coded as in Figure 3.7.
these conditions (Table 3.2), a ground source heat pump that removed 1000 J of heat energy from 1
liter of water would have changed its enthalpy by 0.5% and its temperature by
(1.0 kJ/kg)/(4.18 kJ/kg × K) = 0.24K.
Thus, whether water is pumped to the surface from a depth of 300 meters and passed through
a heat pump, or the heat pump is installed at a depth of 300 meters, the same result would be
obtained.
That result is in striking contrast to the behavior of a system in which water moves from one
set of physical conditions to another set of conditions, and in the process crosses the phase bound-
ary between vapor and liquid. Consider, for example, a water-saturated geothermal reservoir that