Page 63 - Geothermal Energy Renewable Energy and The Environment
P. 63
46 Geothermal Energy: Renewable Energy and the Environment
10,000.0
0°C
1000.0
350°C
400°C
Liquid
100.0 300°C
250°C A
Pressure (bars) 10.0 150°C
A
200°C
1.0 100°C Vapor
B B
20% 40% 60% 80%
0.1 50°C
Liquid + Vapor
0.01
0 500 1000 1500 2000 2500 3000 3500
Enthalpy (kJ/kg)
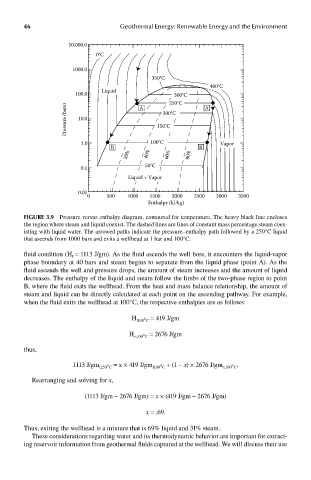
FIGUre 3.9 Pressure versus enthalpy diagram, contoured for temperature. The heavy black line encloses
the region where steam and liquid coexist. The dashed lines are lines of constant mass percentage steam coex-
isting with liquid water. The arrowed paths indicate the pressure–enthalpy path followed by a 250°C liquid
that ascends from 1000 bars and exits a wellhead at 1 bar and 100°C.
fluid condition (H = 1113 J/gm). As the fluid ascends the well bore, it encounters the liquid-vapor
l
phase boundary at 40 bars and steam begins to separate from the liquid phase (point A). As the
fluid ascends the well and pressure drops, the amount of steam increases and the amount of liquid
decreases. The enthalpy of the liquid and steam follow the limbs of the two-phase region to point
B, where the fluid exits the wellhead. From the heat and mass balance relationship, the amount of
steam and liquid can be directly calculated at each point on the ascending pathway. For example,
when the fluid exits the wellhead at 100°C, the respective enthalpies are as follows:
H l100°C = 419 J/gm
H v,100°C = 2676 J/gm
thus,
1113 J/gm l250°C = x × 419 J/gm l100°C + (1 – x) × 2676 J/gm v,100°C .
Rearranging and solving for x,
(1113 J/gm – 2676 J/gm) = x × (419 J/gm – 2676 J/gm)
x = .69.
Thus, exiting the wellhead is a mixture that is 69% liquid and 31% steam.
These considerations regarding water and its thermodynamic behavior are important for extract-
ing reservoir information from geothermal fluids captured at the wellhead. We will discuss their use