Page 65 - Geothermal Energy Renewable Energy and The Environment
P. 65
48 Geothermal Energy: Renewable Energy and the Environment
Rabehl, R. J. 1997. Parameter Estimation and the Use of Catalog Data with TRNSYS, MS thesis, Mechanical
Engineering Department, University of Wisconsin-Madison, Chapter 6.
FUrTher InFormaTIon
Berman, R. G. 1988. “Internally-Consistent Thermodynamic Data for Minerals in the System Na O-
2
K O-CaO-MgO-FeO-Fe O -Al O -SiO -TiO -H O-CO .” Journal of Petrology 29:445–522.
3
2
2
2
2
2
2
3
2
This reference provides a summary of the thermodynamic properties of minerals, as does
the Helgeson et al., 1978 reference mentioned in Table 3.2. These and other tabulations of
the thermodynamic properties of minerals are necessary starting points for calculating
available heat in subsurface reservoirs.
Keenan, J. H., F. G. Keyes, P. G. Hill, and J. G. Moore. 1969. Steam Tables: Thermodynamic
Properties of Water Including Vapor, Liquid and Solid Phases (International Edition—Metric
Units). New York: John Wiley and Sons, Inc.
A basic reference book for the thermodynamic properties of water.
Klotz, I. M., and R. M. Rosenberg. 2008. Chemical Thermodynamics: Basic Concepts and Methods.
Hoboken, NJ: John Wiley and Sons, Inc.
This is a thorough introduction to thermodynamics as applied to chemical processes. A
good reference book for calculation methods.
The International Association for the Properties of Water and Steam
A Web site useful for conducting calculations for the properties of water. Provides links
to NIST and other organizations for obtaining relevant thermodynamic data. http://www.
iapws.org/
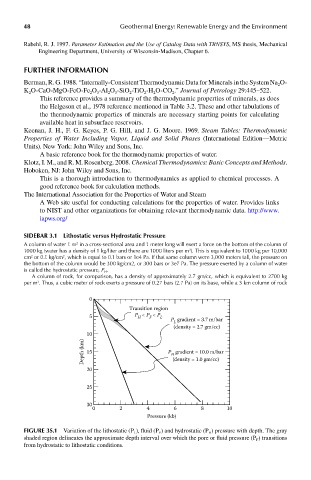
sIdebar 3.1 lithostatic versus hydrostatic pressure
A column of water 1 m in a cross-sectional area and 1 meter long will exert a force on the bottom of the column of
2
1000 kg (water has a density of 1 kg/liter and there are 1000 liters per m ). This is equivalent to 1000 kg per 10,000
3
2
2
cm or 0.1 kg/cm , which is equal to 0.1 bars or 1e4 Pa. If that same column were 3,000 meters tall, the pressure on
the bottom of the column would be 300 kg/cm2, or 300 bars or 3e7 Pa. The pressure exerted by a column of water
is called the hydrostatic pressure, P H .
A column of rock, for comparison, has a density of approximately 2.7 gm/cc, which is equivalent to 2700 kg
per m . Thus, a cubic meter of rock exerts a pressure of 0.27 bars (2.7 Pa) on its base, while a 3 km column of rock
3
0
Transition region
5 P < P < P L P gradient = 3.7 m/bar
H
F
L
(density = 2.7 gm/cc)
10
Depth (km) 15 P gradient = 10.0 m/bar
H
(density = 1.0 gm/cc)
20
25
30
0 2 4 6 8 10
Pressure (kb)
FIGUre 3s.1 Variation of the lithostatic (P L ), fluid (P F ) and hydrostatic (P H ) pressure with depth. The gray
shaded region delineates the approximate depth interval over which the pore or fluid pressure (P F ) transitions
from hydrostatic to lithostatic conditions.