Page 172 - Handbook of Plastics Technologies
P. 172
THERMOSETS
3.42 CHAPTER 3
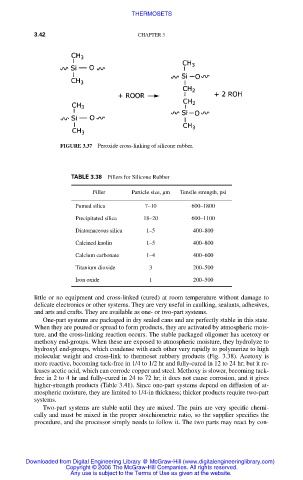
FIGURE 3.37 Peroxide cross-linking of silicone rubber.
TABLE 3.38 Fillers for Silicone Rubber
Filler Particle size, µm Tensile strength, psi
Fumed silica 7–10 600–1800
Precipitated silica 18–20 600–1100
Diatomaceous silica 1–5 400–800
Calcined kaolin 1–5 400–800
Calcium carbonate 1–4 400–600
Titanium dioxide 3 200–500
Iron oxide 1 200–500
little or no equipment and cross-linked (cured) at room temperature without damage to
delicate electronics or other systems. They are very useful in caulking, sealants, adhesives,
and arts and crafts. They are available as one- or two-part systems.
One-part systems are packaged in dry sealed cans and are perfectly stable in this state.
When they are poured or spread to form products, they are activated by atmospheric mois-
ture, and the cross-linking reaction occurs. The stable packaged oligomer has acetoxy or
methoxy end-groups. When these are exposed to atmospheric moisture, they hydrolyze to
hydroxyl end-groups, which condense with each other very rapidly to polymerize to high
molecular weight and cross-link to thermoset rubbery products (Fig. 3.38). Acetoxy is
more reactive, becoming tack-free in 1/4 to 1/2 hr and fully-cured in 12 to 24 hr; but it re-
leases acetic acid, which can corrode copper and steel. Methoxy is slower, becoming tack-
free in 2 to 4 hr and fully-cured in 24 to 72 hr; it does not cause corrosion, and it gives
higher-strength products (Table 3.41). Since one-part systems depend on diffusion of at-
mospheric moisture, they are limited to 1/4-in thickness; thicker products require two-part
systems.
Two-part systems are stable until they are mixed. The pairs are very specific chemi-
cally and must be mixed in the proper stoichiometric ratio, so the supplier specifies the
procedure, and the processor simply needs to follow it. The two parts may react by con-
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2006 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.